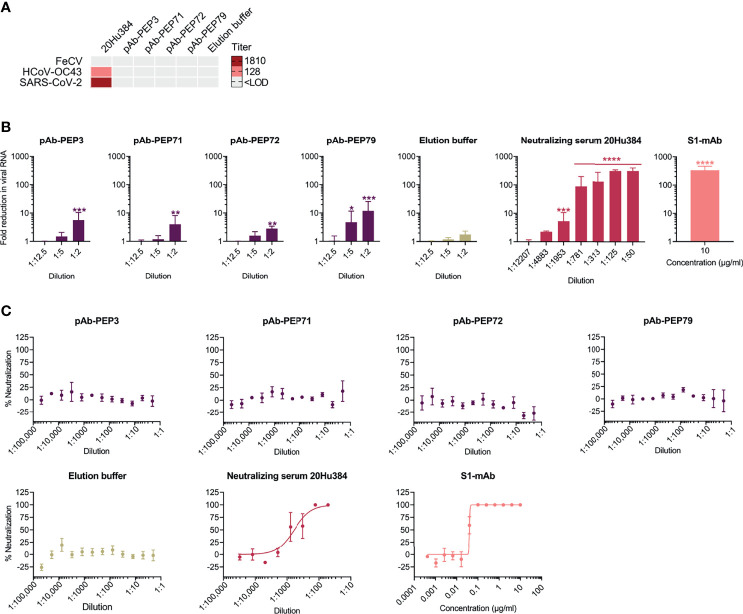
Figure 10.
Virus-neutralizing properties of peptide-purified human antibodies and parent serum 20Hu384. (A) The virus-neutralizing activity was determined by seroneutralization (SN) assay against FeCV in feline enterocytes, HCoV-OC43 in HRT-18 cells, and SARS-CoV-2 in VeroE6 cells. The limit of detection (LOD) was a 1:8 (FeCV) or 1:40 dilution (HCoV-OC43 and SARS-CoV-2). (B, C) Evaluation of the pAbs (starting dilution: 1:2) in a SARS-CoV-2 neutralization assay in Calu-3 cells. (B) Evaluation based on viral load quantification at 24 h p.i. The three right panels show the negative control (elution buffer); parent human serum #20Hu384; and positive control antibody (S1-mAb: SARS-CoV-2 neutralizing anti-S1 antibody). Data are the mean ± SEM of two independent experiments, performed in duplicate. The Y-axis shows the fold reduction in viral RNA versus untreated virus control, based on qRT-PCR analysis of the supernatants at 24 h p.i. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001, ****P ≤ 0.0001 (multiple unpaired t-test with Holm-Šídák correction; treated sample versus untreated virus control). (C) In parallel, virus replication was quantified by immunofluorescence staining for dsRNA at 72 h p.i. The graphs represent the percentage neutralization in function of antibody dilution or concentration.