Abstract
Rationale & Objective
Elevated levels of deoxycholic acid (DCA) are associated with adverse outcomes and may contribute to vascular calcification in patients with chronic kidney disease (CKD). We tested the hypothesis that elevated levels of DCA were associated with increased risks of cardiovascular disease, CKD progression, and death in patients with CKD.
Study Design
Prospective observational cohort study.
Setting & Participants
We included 3,147 Chronic Renal Insufficiency Cohort study participants who had fasting DCA levels. The average age was 59 ± 11 years, 45.3% were women, 40.6% were African American, and the mean estimated glomerular filtration rate was 42.5 ± 16.0 mL/min/1.73 m2.
Predictor
Fasting DCA levels in Chronic Renal Insufficiency Cohort study participants.
Outcomes
Risks of atherosclerotic and heart failure events, end-stage kidney disease (ESKD), and all-cause mortality.
Analytical Approach
We used Tobit regression to identify predictors of DCA levels. We used Cox regression to examine the association between fasting DCA levels and clinical outcomes.
Results
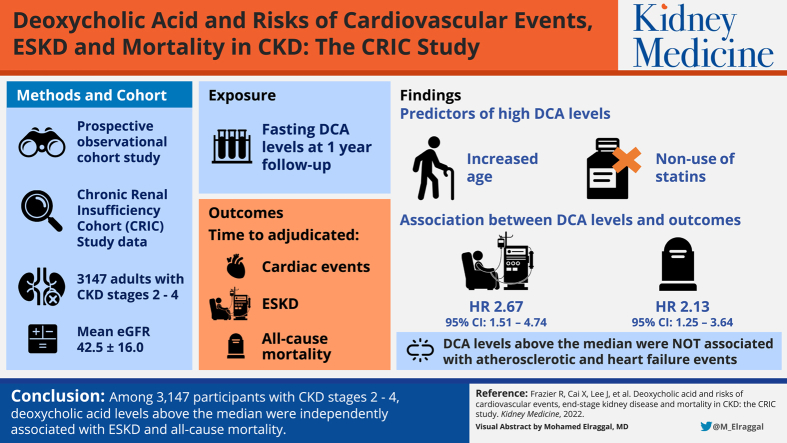
The strongest predictors of elevated DCA levels in adjusted models were increased age and nonuse of statins. The associations between log-transformed DCA levels and clinical outcomes were nonlinear. After adjustment, DCA levels above the median were independently associated with higher risks of ESKD (HR, 2.67; 95% CI, 1.51-4.74) and all-cause mortality (HR, 2.13; 95% CI, 1.25-3.64). DCA levels above the median were not associated with atherosclerotic and heart failure events, and DCA levels below the median were not associated with clinical outcomes.
Limitations
We were unable to measure DCA longitudinally or in urinary or fecal samples, and we were unable to measure other bile acids. We also could not measure many factors that affect DCA levels.
Conclusions
In 3,147 participants with CKD stages 2-4, DCA levels above the median were independently associated with ESKD and all-cause mortality.
Index Words: Cardiovascular disease, chronic kidney disease, deoxycholic acid, end-stage kidney disease, mortality
Graphical abstract
Plain-Language Summary.
Elevated serum levels of deoxycholic acid (DCA), a secondary bile acid, have been associated with vascular calcification in patients with chronic kidney disease (CKD). Using data from the Chronic Renal Insufficiency Cohort study, we tested the associations between elevated DCA levels and increased risks of cardiovascular disease, CKD progression, and death in patients with CKD. DCA was associated with the clinical outcomes in a nonlinear distribution. Using Cox regression, we found that DCA levels above the median were independently associated with higher risks of end-stage kidney disease and mortality but not with atherosclerotic and heart failure events. DCA levels below the median were not significantly associated with clinical outcomes. Additional research is needed to further investigate DCA in CKD.
Individuals with chronic kidney disease (CKD) have an increased prevalence of cardiovascular disease (CVD) and higher risk of death compared with the general population.1,2 With the loss of kidney function, progressive changes in metabolism develop. Human studies report that in CKD, levels of total bile acids increase, possibly because of accelerated hepatic production, increased efflux from hepatocytes, or decreased renal excretion.3, 4, 5 The proportion of secondary bile acids, which are produced by intestinal bacteria from primary bile acids, also increases, likely due to an altered gut microbiome in patients with CKD.6,7 Given that bile acids have important functions besides aiding digestion, including modulation of inflammation, regulation of energy expenditure and metabolism, and effects on immune function, bile acid dysregulation in CKD may have maladaptive effects.
Deoxycholic acid (DCA) is a secondary bile acid derived from cholic acid, a primary bile acid, and studies suggest that DCA levels are elevated in CKD.3,8,9 DCA has been associated with numerous deleterious effects at the cellular level, such as inflammation and immune dysregulation, and risk factors for disease, such as dyslipidemia and decreased insulin sensitivity.10, 11, 12, 13 In addition, experimental data suggest that DCA may exert cardiovascular toxicity by promoting vascular calcification.14 Therefore, elevated DCA levels in patients with CKD may have adverse consequences on cardiovascular and kidney health. To examine the associations between DCA levels and clinical outcomes in patients with CKD stages 2-4, we conducted a prospective observational study within the Chronic Renal Insufficiency Cohort (CRIC) study. We hypothesized that elevated DCA levels would be associated with higher risks of atherosclerotic CVD, heart failure, end-stage kidney disease (ESKD), and all-cause mortality among the 3,147 CRIC study participants.
Methods
CRIC Study
The CRIC study is a multicenter prospective observational cohort of patients with CKD stages 2-4.15,16 The main objective is to investigate risk factors for the development of CVD, progression to ESKD, and mortality in the CKD population. In phase 1 of the CRIC study, 3,939 patients were enrolled across 7 sites from 2003 to 2008. Exclusion criteria included the inability to consent, institutionalization, enrollment in other studies, pregnancy, New York Heart Association class 3-4 heart failure, human immunodeficiency virus infection, cirrhosis, myeloma, polycystic kidney disease, renal cancer, recent chemotherapy or immunosuppressive therapy, organ transplantation, or prior treatment with dialysis for >1 month. All participants gave informed consent, and the protocol was approved by each study site’s institutional review board (University of Pennsylvania IRB protocol 807882). The data underlying this article cannot be shared publicly to protect the privacy of the individuals who participated in the study.
Study Design
We conducted a prospective observational cohort study of 3,147 CRIC study participants, in whom we measured fasting DCA levels in stored serum samples collected at the 1-year follow-up visit, which was the baseline visit for our study. We excluded 419 participants from the baseline to year 1 visit who died, were lost to follow-up, withdrew from the study, or missed the year 1 study visit. From the 3,520 participants who attended the year 1 visit, we excluded 373 participants who did not have available stored samples, who were not fasting at the time of blood draw, or who had progressed to ESKD (Fig S1). Because we assessed the post–year 1 atherosclerotic and heart failure events, participants who had these events before the 1-year follow-up visit were retained in the study.
Exposure
The primary exposure was fasting DCA level at the CRIC study 1-year follow-up visit. Fasting samples were used to control for the postprandial increase in DCA levels.17 Stored frozen serum samples were shipped on dry ice from the CRIC Study Central Laboratory at the University of Pennsylvania to the laboratory of Dr Miyazaki at the University of Colorado. DCA levels were measured using liquid chromatography tandem mass spectrometry, as previously described.18,19 Briefly, 100 μL of human serum was diluted with 300 μL of cold acetonitrile containing 30 ng of D6-DCA (Cambridge Isotope Laboratory) as an internal standard. The solution was passed through a Phree phospholipid removal plate (Phenomenex), and the solvent was evaporated with a nitrogen gas stream. The solute was then redissolved in 100 μL of 10-mM ammonium acetate buffer. The sample was then analyzed with Applied Biosystems 3200 qTRAP liquid chromatography tandem mass spectrometry.14 The intra-assay coefficient of variation for the DCA measurements was 4.3%. Fifteen percent of samples (472 of 3,147) were below the limit of detection, defined by the laboratory as <4 ng/mL. These undetectable results were replaced with the value of 2 ng/mL, half of the lower limit of detection.20,21
Outcomes
The outcome variables investigated were time from the 1-year follow-up visit to adjudicated atherosclerotic and heart failure events, ESKD, and all-cause mortality. Atherosclerotic and heart failure events that occurred before the 1-year follow-up visit were not considered events, and the associated patients were retained in the study to assess post–year 1 outcomes. Atherosclerotic and heart failure events were ascertained every 6 months and adjudicated by medical record review as possible, probable, or definite events. Adjudicated atherosclerotic events were defined as possible, probable, or definite myocardial infarction, probable or definite stroke, or peripheral arterial disease. Adjudicated heart failure events were defined as hospital admission for signs and symptoms of poor cardiac output, and our analysis included both probable and definite adjudicated heart failure events. Progression to ESKD was ascertained every 6 months, confirmed by medical record review, and supplemented with data from the United States Renal Data System. Deaths were confirmed by death certificate.22 Participants were followed until death, loss to follow-up, or administrative end of follow-up in September 2015, with a maximum follow-up time of 11.2 years. All clinical events of interest before the end of follow-up were recorded.
Covariates
Covariates included demographics, cardiovascular risk factors, medication usage, and laboratory values that were routinely collected by the CRIC study. Information about demographics, medical history, and medications was self-reported and obtained at the 1-year follow-up visit. Laboratory tests of blood and urine were measured centrally using standard assays.15,16 Additional information on covariates is presented in Item S1.
Statistical Analysis
Baseline (year 1) characteristics of the CRIC study participants are presented as the total population and by DCA quartiles (Table 1, Table S1). Normally distributed continuous variables are presented as the mean ± standard deviation (SD), whereas skewed continuous variables are presented as the median with interquartile range. Categorical variables are presented as proportions. Statistical differences between quartiles were tested using analysis of variance for continuous variables with normal distributions, Wilcoxon-Mann-Whitney tests for continuous variables with skewed distributions and χ2 tests for categorical variables. DCA was log-transformed in all analyses because of its skewed distribution. Missing covariates were <1.5%. If variables were missing at the year 1 visit, we used data from the initial study visit. We used Tobit regression, which is designed to handle left-censored data, to identify the independent predictors of DCA levels.23 We used Wald χ2 values and P values from the type III analysis of effects to determine the strength of association between log-DCA and the independent variables. In Table S2, we used Pearson correlations to determine the associations between dietary data and DCA.
Table 1.
Baseline Characteristics of CRIC Study Participants by Quartiles of Fasting DCA Level
| Total N = 3,147 | Quartile 1 n = 786 DCA 2.0-23.1 ng/mL | Quartile 2 n = 787 DCA 23.2-68.4 ng/mL | Quartile 3 n = 787 DCA 68.5-148.9 ng/mL | Quartile 4 n = 787 DCA >148.9 ng/mL |
|---|---|---|---|---|
| Age, y | 58 ± 11 | 59 ± 11 | 59 ± 11 | 60 ± 10 |
| Female, N (%) | 358 (45.6) | 361 (45.9) | 341 (43.3) | 367 (46.6) |
| African American, N (%) | 344 (43.8) | 329 (41.8) | 293 (37.2) | 311 (39.5) |
| Hispanic, N (%) | 81 (10.3) | 92 (11.7) | 97 (12.3) | 91 (11.6) |
| Current smoking, N (%) | 113 (14.4) | 97 (12.3) | 81 (10.3) | 88 (11.2) |
| BMI, kg/m2 | 32.1 ± 8.0 | 31.9 ± 7.4 | 32.3 ± 7.9 | 32.4 ± 7.8 |
| Systolic BP, mm Hg | 126 ± 21 | 127 ± 23 | 126 ± 21 | 127 ± 21 |
| Diabetes, N (%) | 403 (51.3) | 377 (47.9) | 332 (42.2) | 398 (50.6) |
| History of CVD, N (%) | 290 (36.9) | 268 (34.1) | 268 (34.1) | 273 (34.7) |
| Total cholesterol, mg/dL | 180.6 ± 43.4 | 183.1 ± 45.2 | 182.3 ± 43.1 | 183.0 ± 43.7 |
| Statin use, N (%) | 506 (64.4) | 466 (59.2) | 440 (55.9) | 432 (54.9) |
| No. of BP medications | 2.6 ± 1.3 | 2.5 ± 1.3 | 2.4 ± 1.3 | 2.4 ± 1.3 |
| eGFR, mL/min/1.73 m2 | 41.0 ± 16.2 | 42.1 ± 16.5 | 43.6 ± 15.7 | 43.3 ± 15.6 |
| Urinary protein, g/24 h | 0.20 (0.07-1.07) | 0.18 (0.07-1.00) | 0.16 (0.07-0.80) | 0.15 (0.07-0.78) |
| Serum albumin, g/dL | 4.02 ± 0.45 | 4.02 ± 0.45 | 4.08 ± 0.41 | 4.08 ± 0.42 |
| Calcium, mg/dL | 9.27 ± 0.53 | 9.29 ± 0.51 | 9.32 ± 0.51 | 9.31 ± 0.51 |
| IL-6, pg/mL | 1.80 (1.13-2.95) | 1.74 (1.08-2.84) | 1.82 (1.05-2.95) | 1.93 (1.21-3.17) |
| CRP, mg/L | 2.63 (1.08-6.87) | 2.34 (1.03-6.00) | 2.53 (0.94-5.74) | 2.38 (1.02-5.99) |
| Phosphate, mg/dL | 3.91 ± 1.24 | 3.85 ± 0.89 | 3.89 ± 1.17 | 3.83 ± 0.81 |
| PTH, pg/mL | 61.9 (39.6-105.0) | 62.0 (40.0-101.0) | 58.1 (40.0-92.4) | 62.0 (39.0-93.7) |
| FGF23, RU/mL | 164.0 (101.2-304.0) | 147.3 (92.6-285.9) | 132.1 (94.3-252.8) | 143.1 (89.9-263.6) |
Note: Continuous variables are presented as mean ± standard deviation for normally distributed data or as median and interquartile range for skewed data. Categorical variables are presented as total number and proportions.
Abbreviations: BMI, body mass index; BP, blood pressure; CRP, C-reactive protein; CVD, cardiovascular disease; DCA, deoxycholic acid; eGFR, estimated glomerular filtration rate; FGF23, fibroblast growth factor 23; IL-6, interleukin 6; PTH, parathyroid hormone.
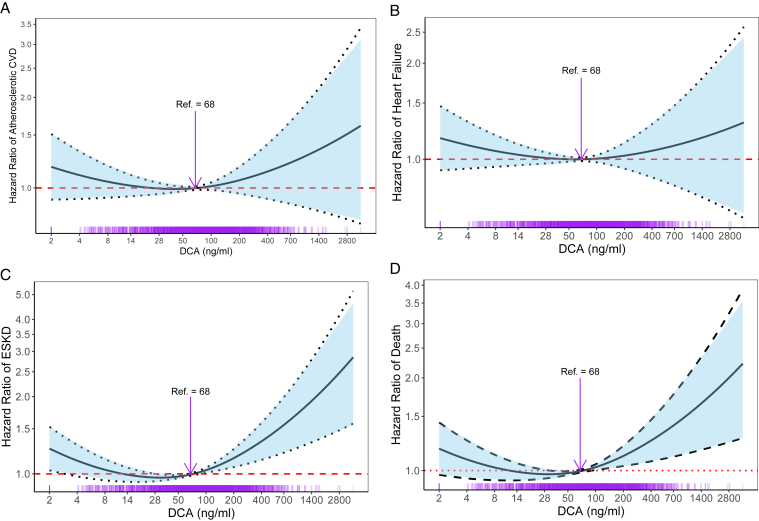
We found that the associations of DCA with the outcome variables were nonlinear. To allow for flexibility in modeling the association between DCA and outcomes, we applied Cox proportional hazards models using penalized cubic splines with 1 knot.24 We determined the optimal number of degrees of freedom and placement of the knot in cubic splines by comparing models with different degrees of freedomand choosing the model with minimum Akaike’s Information Criteria and Bayesian Information Criteria.25,26 The knot was located at the median of DCA value 68.45 ng/mL and was defined as the reference value (hazard ratio [HR] =1.0).
Four models were adjusted sequentially for the following covariates. Model 1 was adjusted for study site, age, sex, race, and Hispanic ethnicity. Model 2 was adjusted for the covariates in model 1, plus renal and cardiovascular risk factors (estimated glomerular filtration rate, log-transformed urinary protein, diabetes, systolic blood pressure, the number of antihypertensive medications, current smoking, history of CVD, total cholesterol, and statin use). Model 3 was adjusted for factors in model 2, plus log-transformed interleukin-6 and log-transformed C-reactive protein. Model 4 was adjusted for factors in model 3, plus log-transformed fibroblast growth factor-23, log-transformed parathyroid hormone, phosphate, calcium, and albumin. In Table S3, model 5 adjusts for factors in model 4, plus age of the DCA sample.
In Table 2, we report HRs and 95% confidence intervals (CIs) per 1 SD of log-transformed DCA compared with the HR at the median DCA value (68.45 ng/mL). Figure 1 presents the HRs with 95% CIs from model 4 for each outcome. Any portion of the curve above the y-scale reference line of 1.0 was considered statistically significant. The DCA values on the x-axis were back-transformed from the SD of log-transformed DCA values to their original scale in the unit of DCA (ng/mL). The rug plot at the bottom of Figure 1 displays the number of measurements.27
Table 2.
Associations of Fasting DCA Level With Clinical Outcomes
| Below DCA Median, <68.45 ng/mL | Above DCA Median, >68.45 ng/mL | |
|---|---|---|
| Atherosclerotic events | ||
| Events/total number | 261/1,574 | 251/1,573 |
| Mean follow-up time (± standard deviation), y | 6.9 ± 3.3 | 6.7 ± 3.3 |
| Hazard ratio (95% CI) per SD of log-transformed DCA | ||
| Unadjusted | 0.88 (0.56-1.37) | 1.83 (0.89-3.74) |
| Model 1a | 0.82 (0.53-1.28) | 1.49 (0.72-3.08) |
| Model 2b | 0.91 (0.58-1.43) | 1.48 (0.72-3.01) |
| Model 3c | 0.90 (0.57-1.42) | 1.58 (0.77-3.22) |
| Model 4d | 0.88 (0.56-1.40) | 1.52 (0.74-3.12) |
| Heart failure events | ||
| Events/total number | 303/1,574 | 272/1,573 |
| Mean follow-up time (± standard deviation), y | 7.1 ± 3.2 | 7.0 ± 3.2 |
| Hazard ratio (95% CI) per SD of log-transformed DCA | ||
| Unadjusted | 0.75 (0.50-1.13) | 1.58 (0.80-3.14) |
| Model 1a | 0.74 (0.49-1.12) | 1.40 (0.70-2.80) |
| Model 2b | 0.86 (0.56-1.31) | 1.40 (0.71-2.73) |
| Model 3c | 0.81 (0.53-1.24) | 1.29 (0.66-2.53) |
| Model 4d | 0.82 (0.54-1.27) | 1.22 (0.63-2.38) |
| ESKD events | ||
| Events/total number | 456/1,574 | 373/1,573 |
| Mean follow-up time (± standard deviation), y | 6.9 ± 3.3 | 7.0 ± 3.1 |
| Hazard ratio (95% CI) per SD of log-transformed DCA | ||
| Unadjusted | 0.58 (0.41-0.81) | 1.24 (0.69-2.23) |
| Model 1a | 0.62 (0.44-0.88) | 1.33 (0.74-2.41) |
| Model 2b | 0.76 (0.54-1.08) | 2.12 (1.18-3.82) |
| Model 3c | 0.79 (0.55-1.12) | 2.21 (1.23-3.99) |
| Model 4d | 0.98 (0.68-1.40) | 2.67 (1.51-4.74) |
| All-cause mortality events | ||
| Events/total number | 408/1,574 | 411/1,573 |
| Mean follow-up time (± standard deviation), y | 8.1 ± 2.6 | 7.9 ± 2.6 |
| Hazard ratio (95% CI) per SD of log-transformed DCA | ||
| Unadjusted | 0.88 (0.63-1.24) | 2.36 (1.37-4.07) |
| Model 1a | 0.81 (0.58-1.15) | 2.05 (1.17-3.56) |
| Model 2b | 1.03 (0.72-1.46) | 2.12 (1.24-3.64) |
| Model 3c | 0.98 (0.69-1.40) | 2.11 (1.23-3.64) |
| Model 4d | 1.00 (0.70-1.43) | 2.13 (1.25-3.64) |
Abbreviations: CI, confidence interval; DCA, deoxycholic acid; ESKD, end-stage kidney disease; SD, standard deviation.
Model 1 stratified by study site and adjusted for age, sex, African American race, and Hispanic ethnicity.
Model 2 adjusted for model 1 + estimated glomerular filtration rate, log urinary protein, diabetes, systolic blood pressure, number of antihypertensive medications, current smoking, history of cardiovascular disease, total cholesterol, and statin use.
Model 3 adjusted for model 2 + log Interleukin-6 and log C-reactive protein.
Model 4 adjusted for model 3 + log fibroblast growth factor 23, log parathyroid hormone, phosphate, calcium, and albumin.
Figure 1.
Adjusted hazard ratios for atherosclerotic CVD, heart failure events, ESKD, and mortality according to DCA levels. Adjusted hazard ratios with 95% CIs for (A) atherosclerotic CVD, (B) heart failure events, (C) ESKD, and (D) mortality according to DCA levels. The curve above the y-scale reference line of 1.0 is statistically significant. Models were adjusted for the covariates in model 4, including age, sex, African American race, Hispanic ethnicity, eGFR, log urinary protein, diabetes, SBP, number of antihypertensive medications, current smoking, history of cardiovascular disease, total cholesterol, statin use, log IL-6, log CRP, log FGF23, log PTH, phosphate, calcium, and albumin. The DCA values on the x-axis were back-transformed per 1 SD of log-transformed DCA values. The rug plot at the bottom of the figures displays the number of measurements. Abbreviations: CRP, C-reactive protein; CVD, cardiovascular disease; DCA, deoxycholic acid; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; FGF23, fibroblast growth factor 23; IL-6, interleukin 6; PTH, parathyroid hormone; SBP, systolic blood pressure; SD, standard deviation.
We calculated Schoenfeld residuals in the fully adjusted models among the individuals with DCA values below the median and above the median. We verified that there was no violation of the proportional hazards assumption for DCA in all outcome models. All analyses were performed using Survival, SmoothHR, and Splines packages in R, version 3.4.4, and SAS version 9.4. Two-sided P values < 0.05 were considered statistically significant.
Results
Baseline Characteristics
Among the 3,147 participants with fasting DCA levels at the 1-year follow-up visit, which was the baseline visit for the current analyses, average age was 59 ± 11 years, 45.3% were women, 40.6% were African American, and 48.0% had diabetes (Table S1). The average estimated glomerular filtration rate was 42.5 ± 16.0 mL/min/1.73 m2. Several participant characteristics varied by DCA quartile (Table 1). Higher DCA levels were associated with increased age, lower percentage of African American participants, lower statin use, higher estimated glomerular filtration rate, higher albumin, higher interleukin-6, and lower fibroblast growth factor-23 levels. There was no significant association of DCA quartiles with sex, current smoking, body mass index, systolic blood pressure, history of CVD, total cholesterol, number of blood pressure medications, urinary protein, calcium, C-reactive protein, phosphate, or parathyroid hormone. Dietary protein and fat were also not significantly associated with DCA levels (Table S2). After multivariable adjustment of the Tobit regression model, the 2 variables most strongly associated with higher DCA levels were increased age and nonuse of statins (Table S4).
Associations of Fasting DCA Level With Adjudicated Atherosclerotic and Heart Failure Events
The associations of DCA with all outcomes were nonlinear (Fig 1). Table 2 shows the associations between DCA and atherosclerotic and heart failure events. In the group with DCA levels below the median (68.45 ng/mL), there were 261 (16.6%) atherosclerotic events with a mean follow-up time of 6.9 years. Among individuals with DCA levels above the median, there were 251 (16.0%) atherosclerotic events with a mean follow-up time of 6.7 years. In both groups, DCA levels were not associated with atherosclerotic events in the unadjusted analysis or the adjusted models (HR, 0.88 and 95% CI, 0.56-1.40 for model 4 with DCA below the median; HR, 1.52 and 95% CI, 0.74-3.12 for model 4 with DCA above the median; Fig 1A). In the heart failure analysis, there were 303 (19.3%) events in those with DCA values below the median with a mean follow-up time of 7.1 years. Among individuals with DCA values above the median, there were 272 (17.3%) heart failure events with a mean follow-up time of 7.0 years. Similarly, there were no associations between DCA values and heart failure events in the unadjusted and adjusted analyses (HR, 0.82 and 95% CI, 0.54-1.27 in model 4 with DCA below the median; HR, 1.22 and 95% CI, 0.63-2.38 in model 4 with DCA above the median; Fig 1B).
Association of Fasting DCA Level With ESKD
Table 2 shows the association of fasting DCA level with progression to ESKD. Among participants with baseline DCA levels below the median, there were 456 (29.0%) events with a mean follow-up time of 6.9 years. In this population with DCA levels below the median, DCA levels closer to the median value were associated with lower risk of progression to ESKD (HR, 0.58 and 95% CI, 0.41-0.81 in the unadjusted analysis; HR, 0.62 and 95% CI, 0.44-0.88 in model 1). However, this association was no longer significant after adjustment for CKD and CVD risk factors (HR, 0.98 and 95% CI, 0.68-1.40 in model 4, Fig 1C).
Among individuals with DCA values above the median, there were 373 (23.7%) events with a mean follow-up time of 7.0 years. In the unadjusted analysis and model 1, there was no significant association between DCA and ESKD. However, after adjustment for CKD and CVD risk factors, the association became significant. Adjustments for bone and mineral factors in model 4 also strengthened this association (HR, 2.67 and 95% CI, 1.51-4.74 in model 4).
Associations of Fasting DCA Level With All-Cause Mortality
Table 2 shows the associations of DCA with all-cause mortality. Among individuals with DCA values below the median, there were 408 (25.9%) total deaths with a mean follow-up time of 8.1 years. Low DCA values were not associated with all-cause mortality in the unadjusted analysis or in any of the adjusted models (HR, 1.00 and 95% CI, 0.70-1.43 in model 4, Fig 1D).
Among individuals with DCA values above the median, there were 411 (26.1%) deaths with a mean follow-up time of 7.9 years. High DCA levels were significantly associated with all-cause mortality in the unadjusted analysis and all of the additional models, independent of demographics, renal risk factors, CVD risk factors, inflammatory markers, and markers of mineral metabolism (HR, 2.13 and 95% CI, 1.25-3.64 in model 4).
We also investigated the age of the DCA sample on clinical outcomes. We found that adjusting for DCA sample age did not significantly affect the association between DCA and atherosclerotic events, heart failure events, ESKD, or mortality (model 5 in Table S3).
Discussion
Our study found that in 3,147 participants with CKD stages 2-4, the associations between DCA levels and heart failure and atherosclerotic events, progression to ESKD, and mortality were nonlinear. In multivariable-adjusted models, DCA levels above the median were independently associated with ESKD and all-cause mortality, whereas those below the median were not associated with clinical outcomes after adjustment. Our findings, which will require validation in future studies, provide support for the emerging evidence of the complex effects of bile acids on human health.
Bile acids primarily aid in digestion, although there is growing recognition of their numerous effects throughout the body. Primary bile acids, such as cholic acid, are formed from cholesterol precursors in the liver and are excreted into the intestines to aid in lipid digestion, where a portion is metabolized by intestinal bacteria into secondary bile acids, such as DCA. Most bile acids are reabsorbed in the distal ileum and returned to the liver as part of the enterohepatic circulation. However, a low level remains in the systemic circulation. Many factors are thought to alter the level and composition of bile acids, including diet, medications, and the gut microbiome.6,28,29 A high-fat “Western” diet and alcohol use may increase DCA levels.30,31 CKD may also alter bile acid levels. Prior research has indicated that those with CKD have elevated total bile acids compared with those without CKD and have an increased DCA to cholic acid ratio.3,8,9
We found that among individuals with CKD stages 2-4, elevated DCA levels were most strongly associated with increased age and nonuse of statins after multivariable adjustment. Statins competitively inhibit the activity of 3-hydroxy-3-methyl-glutaryl-CoA reductase, the rate-limiting step in cholesterol synthesis, resulting in lowered cellular cholesterol concentration.32 Bile acids are formed from cholesterol precursors; thus, reducing cholesterol may reduce bile acid synthesis and quantity. The effect of age on DCA levels is unclear, with contradictory reports in the literature.33,34 In contrast to prior studies, we did not find an independent relationship between kidney function and DCA levels.3,8,9 Additional research is needed to examine DCA levels across the full spectrum of kidney function.
In our study, DCA levels were associated with clinical outcomes in a nonlinear or biphasic pattern. This distribution suggests that moderate DCA concentrations may be optimal, whereas more extreme levels may have deleterious effects. Many biological molecules exhibit this biphasic dose-response curve.35,36 With some substances, a minimum threshold is needed for normal function and yet high levels cause toxicity, creating a middle range of ideal functioning. Because DCA and other bile acids, in addition to their role in digestion, have hormone-like properties with systemic physiologic effects, we speculate that DCA may be harmful at high levels, but some level of DCA above a minimal threshold may be advantageous. Future studies will need to test this hypothesis and further investigate the optimal range of DCA levels.
Prior studies showing that DCA is associated with both beneficial and harmful outcomes in the body support our assertion of an optimal threshold level of DCA. DCA has detergent properties that assist the digestion of dietary lipids and can also affect cellular lipid bilayers. DCA has been shown to disrupt gastrointestinal mucosal barriers, leading to cellular damage and inflammation.37 DCA also generates reactive oxygen species, causing DNA damage and cellular apoptosis, and stimulates the production of proinflammatory, procalcification, and protumorigenic factors.10,11,38,39 In addition, both animal and human studies suggest that elevated DCA levels may contribute to vascular calcification, which may worsen cardiovascular and kidney disease.3,14,40 However, research also suggests that DCA may have favorable effects through the activation of its receptor, the farsenoid X receptor. Farsenoid X receptor activation decreases gluconeogenesis, increases glycolysis, induces hepatic lipoprotein clearance, and reduces fatty acid synthesis, leading to improved glucose tolerance, insulin sensitivity, and lipid profiles.41, 42, 43, 44 In addition, in the kidney, farsenoid X receptor activation has been associated with reduced diabetic nephropathy and renal fibrosis.45, 46, 47 Thus at low DCA levels, reduced farsenoid X receptor stimulation may worsen metabolic syndrome and kidney disease, but at high levels, DCA may cause cellular damage and vascular calcification and may hasten CKD progression. Additional studies are needed to validate our findings and to further examine the exact mechanisms of beneficial and harmful effects of DCA on kidney function.
In this study, we found that elevated DCA was associated with a higher risk of all-cause mortality but not with atherosclerotic or heart failure events. There are 2 possibilities that may reconcile these disparate findings. First, there may not be a true association of DCA with CVD, and our findings of a significant association of DCA with mortality suggest that elevated DCA may contribute to mortality through mechanisms other than CVD. Second, DCA may be associated with CVD events but not heart failure or atherosclerotic events. Patients with CKD are susceptible to medial arterial calcification, which is associated with arrhythmias and sudden cardiac death instead of atherosclerotic events or heart failure.48,49 Future studies are needed to confirm or refute our findings.
There are several strengths of our study. We used data from the CRIC Study, which is a large, prospective cohort of a diverse patient population with CKD stages 2-4. Given the long follow-up time and many participants in the cohort, we were able to assess clinically-relevant outcomes associated with DCA levels. The CRIC Study uses standardized data collection methods, collects many variables, and clinical outcomes are adjudicated. We were also able to measure the fasting DCA levels on most of the CRIC Study participants. However, there are also limitations. We do not have longitudinal data on DCA levels, and thus we do not know the variability of DCA in each participant or how DCA levels over time affect clinical outcomes. We were unable to measure other bile acids and thus cannot make conclusions about the total bile acid pool or ratios of bile acids. We were only able to measure serum DCA and not urinary or fecal DCA levels and thus may have an incomplete understanding of patients’ DCA metabolism. We were unable to measure many factors that may affect DCA levels, such as liver disease, alcohol consumption, the gut microbiome, fat malabsorption, certain medications, or complete dietary intake. In addition, the cause of death of many participants was unable to be determined, and outcomes of interest such as sudden cardiac death and arrhythmias were not available.
In conclusion, our study suggests that high DCA levels are associated with the risk of ESKD and all-cause mortality in patients with CKD. Although there are several possible biological mechanisms linking DCA to poor clinical outcomes, there is still much that is unknown about DCA in kidney disease. Further research is needed to validate our findings and to determine the exact role of DCA levels in the pathogenesis of cardiovascular and kidney disease and the clinical effects in patients with CKD.
Article Information
CRIC Study Investigators
Lawrence J. Appel, MD, MPH, Harold I. Feldman, MD, MSCE, Alan S. Go, MD, Jiang He, MD, PhD, James P. Lash, MD, Robert G. Nelson, MD, PhD, MS, Mahboob Rahman, MD, Panduranga S. Rao, MD, Vallabh O. Shah, PhD, MS, Raymond R. Townsend, MD, and Mark L. Unruh, MD, MS.
Authors’ Full Names and Academic Degrees
Rebecca Frazier, MD, Xuan Cai, MS, Jungwha Lee, PhD, Joshua D. Bundy, PhD, MPH, Anna Jovanovich, MD, Jing Chen, MD, MMSc, MSc, Rajat Deo, MD, MTR, James P. Lash, MD, Amanda Hyre Anderson, PhD, MPH, Alan S. Go, MD, Harold I. Feldman, MD, MSCE, Tariq Shafi, MBBS, MHS, Eugene P. Rhee, MD, Makoto Miyazaki, PhD, Michel Chonchol, MD, and Tamara Isakova, MD, MMSc, on behalf of the CRIC Study Investigators.
Authors’ Contributions
Study design: TI, MC, MM, AJ; data collection: JPL, RD, AHA, JC, ASG, TS, HIF; data analysis: XC, JL, MM; data interpretation: TI, MC, AJ, ER, RF, JDB; figure creation: XC. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
This work was supported by the George M. O’Brien Kidney Research Center at Northwestern University (NU-GoKIDNEY; P30DK114857) from the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). RF was supported by grant T32DK108738 from the NIDDK. TI was supported by grants R01DK102438, R01DK110087, and U01DK099930 from the NIDDK and K24HL150235 from the National Heart, Lung, and Blood Institute.
Funding for the CRIC Study was obtained from grants U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902 under a cooperative agreement from the NIDDK. In addition, this study was supported in part by the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award National Institutes of Health/National Center for Advancing Translational Sciences (UL1TR000003), the Johns Hopkins Institute for Clinical and Translational Research (UL1TR000424), University of Maryland General Clinical Research Center (M01RR-16500), Clinical and Translational Science Collaborative of Cleveland (UL1TR000439), Michigan Institute for Clinical and Health Research (UL1TR000433), University of Illinois at Chicago Clinical and Translational Science Awards (UL1RR029879), Tulane Center of Biomedical Research Excellence for Clinical and Translational Research in Cardiometabolic Diseases (P20GM109036), and Kaiser Permanente National Institutes of Health/National Center for Research Resources University of California San Francisco Clinical and Translational Science Institute (UL1RR024131).
The funders of this study did not have any role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
Financial Disclosure
Dr Isakova reports consulting honorariums from Akebia Therapeutics, Inc, Kyowa Kirin Co, Ltd, and LifeSci Capital, LLC. Dr Feldman reports relationships with Kyowa Hakko Kirin Co, Ltd, American Journal of Kidney Disease, InMed Physicians, and DLA Piper LLP. Drs Anderson, Go, and Lash report grants from the NIH during the conduct of the study. Dr Go reports grants from AstraZeneca. The remaining authors declare that they have no relevant financial interests.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Prior Presentation
Part of this work was presented as a poster presentation at the American Society of Nephrology Scientific Session on November 7, 2019 in Washington, DC.
Peer Review
Received March 31, 2021. Evaluated by 2 external peer reviewers, with direct editorial input by the Statistical Editor, an Associate Editor, and the Editor-in-Chief. Accepted in revised form September 26, 2021.
Footnotes
Complete author and article information provided before references.
Figure S1: Study population derived from the total CRIC Study population.
Item S1: Supplementary Methods.
Table S1: Characteristics of all CRIC Study participants who attended the year 1 visit and of the participants included in the study population.
Table S2: Associations of dietary protein and fat with log-DCA.
Table S3: Associations of fasting DCA level with clinical outcomes, adjusted for age of sample.
Table S4: Associations between log-DCA and DCA predictors.
Supplementary Material
Figure S1; Item S1; Tables S1-S4.
References
- 1.Saran R., Robinson B., Abbott K.C., et al. US Renal Data System 2018 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2019;73(3 suppl 1):A7–A8. doi: 10.1053/j.ajkd.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foley R.N., Murray A.M., Li S., et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol. 2005;16(2):489–495. doi: 10.1681/asn.2004030203. [DOI] [PubMed] [Google Scholar]
- 3.Miyazaki M., Miyazaki-Anzai S., Masuda M., Kremoser C. Deoxycholic acid contributes to chronic kidney disease-dependent vascular calcification. Circulation. 2013;128(suppl 22):A14937. [Google Scholar]
- 4.Chu L., Zhang K., Zhang Y., Jin X., Jiang H. Mechanism underlying an elevated serum bile acid level in chronic renal failure patients. Int Urol Nephrol. 2015;47(2):345–351. doi: 10.1007/s11255-014-0901-0. [DOI] [PubMed] [Google Scholar]
- 5.Gai Z., Chu L., Hiller C., et al. Effect of chronic renal failure on the hepatic, intestinal, and renal expression of bile acid transporters. Am J Physiol Renal Physiol. 2014;306(1):F130–F137. doi: 10.1152/ajprenal.00114.2013. [DOI] [PubMed] [Google Scholar]
- 6.Ramezani A., Raj D.S. The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol. 2014;25(4):657–670. doi: 10.1681/asn.2013080905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaziri N.D., Wong J., Pahl M., et al. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013;83(2):308–315. doi: 10.1038/ki.2012.345. [DOI] [PubMed] [Google Scholar]
- 8.Jimenez F., Monte M.J., El-Mir M.Y., Pascual M.J., Marin J.J. Chronic renal failure-induced changes in serum and urine bile acid profiles. Dig Dis Sci. 2002;47(11):2398–2406. doi: 10.1023/a:1020575001944. [DOI] [PubMed] [Google Scholar]
- 9.Marecková O., Skála I., Marecek Z., et al. Bile composition in patients with chronic renal insufficiency. Nephrol Dial Transplant. 1990;5(6):423–425. doi: 10.1093/ndt/5.6.423. [DOI] [PubMed] [Google Scholar]
- 10.Farhana L., Nangia-Makker P., Arbit E., et al. Bile acid: a potential inducer of colon cancer stem cells. Stem Cell Res Ther. 2016;7(1):181. doi: 10.1186/s13287-016-0439-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshimoto S., Loo T.M., Atarashi K., et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499(7456):97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 12.Haeusler R.A., Astiarraga B., Camastra S., Accili D., Ferrannini E. Human insulin resistance is associated with increased plasma levels of 12α-hydroxylated bile acids. Diabetes. 2013;62(12):4184–4191. doi: 10.2337/db13-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brufau G., Stellaard F., Prado K., et al. Improved glycemic control with colesevelam treatment in patients with type 2 diabetes is not directly associated with changes in bile acid metabolism. Hepatology. 2010;52(4):1455–1464. doi: 10.1002/hep.23831. [DOI] [PubMed] [Google Scholar]
- 14.Jovanovich A., Isakova T., Block G., et al. Deoxycholic acid, a metabolite of circulating bile acids, and coronary artery vascular calcification in CKD. Am J Kidney Dis. 2018;71(1):27–34. doi: 10.1053/j.ajkd.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lash J.P., Go A.S., Appel L.J., et al. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4(8):1302–1311. doi: 10.2215/cjn.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feldman H.I., Appel L.J., Chertow G.M., et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: design and methods. J Am Soc Nephrol. 2003;14(7 Suppl 2):S148–S153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 17.Bathena S.P., Thakare R., Gautam N., et al. Urinary bile acids as biomarkers for liver diseases I. Stability of the baseline profile in healthy subjects. Toxicol Sci. 2015;143(2):296–307. doi: 10.1093/toxsci/kfu227. [DOI] [PubMed] [Google Scholar]
- 18.Ando M., Kaneko T., Watanabe R., et al. High sensitive analysis of rat serum bile acids by liquid chromatography/electrospray ionization tandem mass spectrometry. J Pharm Biomed Anal. 2006;40(5):1179–1186. doi: 10.1016/j.jpba.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Miyazaki-Anzai S., Masuda M., Levi M., Keenan A.L., Miyazaki M. Dual activation of the bile acid nuclear receptor FXR and G-protein-coupled receptor TGR5 protects mice against atherosclerosis. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0108270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hornung R.W., Reed L.D. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5(1):46–51. [Google Scholar]
- 21.MahmoudianDehkordi S., Arnold M., Nho K., et al. Altered bile acid profile associates with cognitive impairment in Alzheimer’s disease-an emerging role for gut microbiome. Alzheimers Dement. 2019;15(1):76–92. doi: 10.1016/j.jalz.2018.07.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bundy J.D., Cai X., Mehta R.C., et al. Serum calcification propensity and clinical events in CKD. Clin J Am Soc Nephrol. 2019;14(11):1562–1571. doi: 10.2215/cjn.04710419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tobin J. Estimation of relationships for limited dependent variables. Econometrica. 1958;26(1):24–36. [Google Scholar]
- 24.Ku E., Hsu R.K., Tuot D.S., et al. Magnitude of the difference between clinic and ambulatory blood pressures and risk of adverse outcomes in patients with chronic kidney disease. J Am Heart Assoc. 2019;8(9) doi: 10.1161/jaha.118.011013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meira-Machado L., Cadarso-Suárez C., Gude F., Araújo A. smoothHR: an R package for pointwise nonparametric estimation of hazard ratio curves of continuous predictors. Comput Math Methods Med. 2013;2013:745742. doi: 10.1155/2013/745742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dziak J.J., Coffman D.L., Lanza S.T., Li R., Jermiin L.S. Sensitivity and specificity of information criteria. Brief Bioinform. 2020;21(2):553–565. doi: 10.1093/bib/bbz016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Núñez J., Bayés-Genís A., Zannad F., et al. Long-term potassium monitoring and dynamics in heart failure and risk of mortality. Circulation. 2018;137(13):1320–1330. doi: 10.1161/circulationaha.117.030576. [DOI] [PubMed] [Google Scholar]
- 28.Rafter J.J., Child P., Anderson A.M., Alder R., Eng V., Bruce W.R. Cellular toxicity of fecal water depends on diet. Am J Clin Nutr. 1987;45(3):559–563. doi: 10.1093/ajcn/45.3.559. [DOI] [PubMed] [Google Scholar]
- 29.Braunlin W., Zhorov E., Guo A., et al. Bile acid binding to sevelamer HCl. Kidney Int. 2002;62(2):611–619. doi: 10.1046/j.1523-1755.2002.00459.x. [DOI] [PubMed] [Google Scholar]
- 30.McGarr S.E., Ridlon J.M., Hylemon P.B. Diet, anaerobic bacterial metabolism, and colon cancer: a review of the literature. J Clin Gastroenterol. 2005;39(2):98–109. [PubMed] [Google Scholar]
- 31.Gong X., Zhang Q., Ruan Y., Hu M., Liu Z., Gong L. Chronic alcohol consumption increased bile acid levels in enterohepatic circulation and reduced efficacy of irinotecan. Alcohol Alcohol. 2020;55(3):264–277. doi: 10.1093/alcalc/agaa005. [DOI] [PubMed] [Google Scholar]
- 32.Corsini A., Maggi F.M., Catapano A.L. Pharmacology of competitive inhibitors of HMG-CoA reductase. Pharmacol Res. 1995;31(1):9–27. doi: 10.1016/1043-6618(95)80042-5. [DOI] [PubMed] [Google Scholar]
- 33.Xie G., Wang Y., Wang X., et al. Profiling of serum bile acids in a healthy Chinese population using UPLC-MS/MS. J Proteome Res. 2015;14(2):850–859. doi: 10.1021/pr500920q. [DOI] [PubMed] [Google Scholar]
- 34.Frommherz L., Bub A., Hummel E., et al. Age-related changes of plasma bile acid concentrations in healthy adults--results from the cross-sectional KarMeN Study. PLoS One. 2016;11(4) doi: 10.1371/journal.pone.0153959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis J.M., Svendsgaard D.J. U-shaped dose-response curves: their occurrence and implications for risk assessment. J Toxicol Environ Health. 1990;30(2):71–83. doi: 10.1080/15287399009531412. [DOI] [PubMed] [Google Scholar]
- 36.Calabrese E.J., Baldwin L.A. Hormesis: U-shaped dose responses and their centrality in toxicology. Trends Pharmacol Sci. 2001;22(6):285–291. doi: 10.1016/s0165-6147(00)01719-3. [DOI] [PubMed] [Google Scholar]
- 37.Liu L., Dong W., Wang S., et al. Deoxycholic acid disrupts the intestinal mucosal barrier and promotes intestinal tumorigenesis. Food Funct. 2018;9(11):5588–5597. doi: 10.1039/c8fo01143e. [DOI] [PubMed] [Google Scholar]
- 38.Payne C.M., Weber C., Crowley-Skillicorn C., et al. Deoxycholate induces mitochondrial oxidative stress and activates NF-kappaB through multiple mechanisms in HCT-116 colon epithelial cells. Carcinogenesis. 2007;28(1):215–222. doi: 10.1093/carcin/bgl139. [DOI] [PubMed] [Google Scholar]
- 39.Proudfoot D., Skepper J.N., Hegyi L., Bennett M.R., Shanahan C.M., Weissberg P.L. Apoptosis regulates human vascular calcification in vitro: evidence for initiation of vascular calcification by apoptotic bodies. Circ Res. 2000;87(11):1055–1062. doi: 10.1161/01.res.87.11.1055. [DOI] [PubMed] [Google Scholar]
- 40.Duan X., Zhou Y., Teng X., Tang C., Qi Y. Endoplasmic reticulum stress-mediated apoptosis is activated in vascular calcification. Biochem Biophys Res Commun. 2009;387(4):694–699. doi: 10.1016/j.bbrc.2009.07.085. [DOI] [PubMed] [Google Scholar]
- 41.Cipriani S., Mencarelli A., Palladino G., Fiorucci S. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. J Lipid Res. 2010;51(4):771–784. doi: 10.1194/jlr.M001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int Suppl. 2009;(113):S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 43.de Aguiar Vallim T.Q., Tarling E.J., Edwards P.A. Pleiotropic roles of bile acids in metabolism. Cell Metab. 2013;17(5):657–669. doi: 10.1016/j.cmet.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y., Lee F.Y., Barrera G., et al. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci U S A. 2006;103(4):1006–1011. doi: 10.1073/pnas.0506982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X.X., Jiang T., Shen Y., et al. Diabetic nephropathy is accelerated by farnesoid X receptor deficiency and inhibited by farnesoid X receptor activation in a type 1 diabetes model. Diabetes. 2010;59(11):2916–2927. doi: 10.2337/db10-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao K., He J., Zhang Y., et al. Activation of FXR protects against renal fibrosis via suppressing Smad3 expression. Sci Rep. 2016;6:37234. doi: 10.1038/srep37234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang T., Wang X.X., Scherzer P., et al. Farnesoid X receptor modulates renal lipid metabolism, fibrosis, and diabetic nephropathy. Diabetes. 2007;56(10):2485–2493. doi: 10.2337/db06-1642. [DOI] [PubMed] [Google Scholar]
- 48.Goodman W.G., London G., Amann K., et al. Vascular calcification in chronic kidney disease. Am J Kidney Dis. 2004;43(3):572–579. doi: 10.1053/j.ajkd.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 49.Guérin A.P., Pannier B., Métivier F., Marchais S.J., London G.M. Assessment and significance of arterial stiffness in patients with chronic kidney disease. Curr Opin Nephrol Hypertens. 2008;17(6):635–641. doi: 10.1097/mnh.0b013e32830dcd5c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1; Item S1; Tables S1-S4.