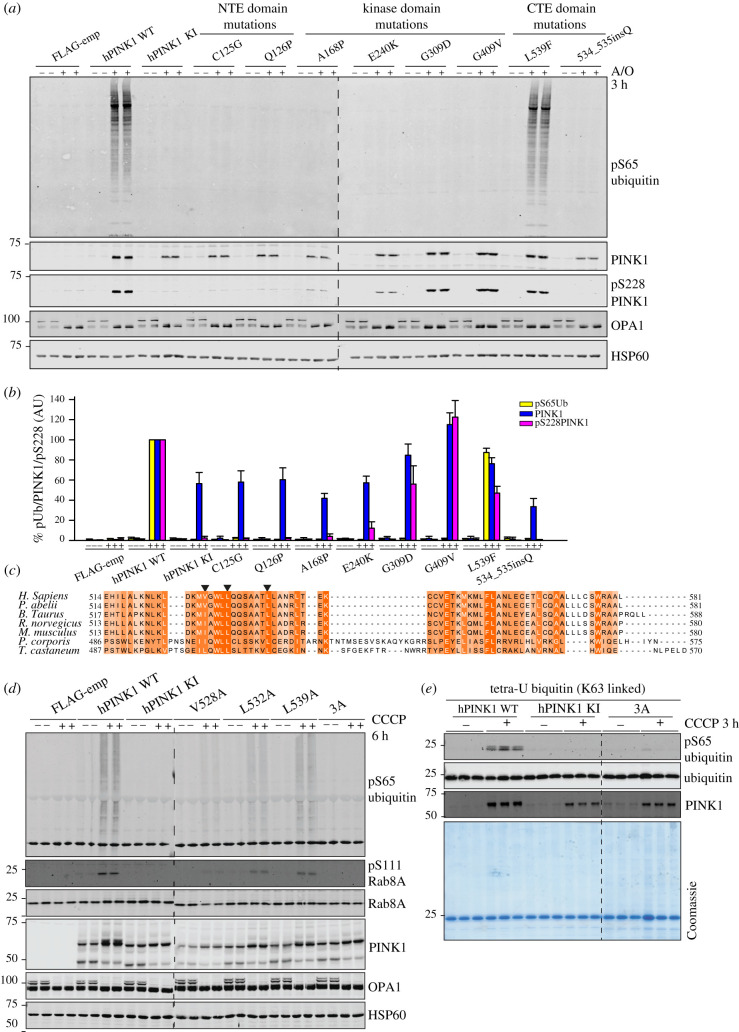
Figure 4.
Mutational analysis confirms the critical role of NTE and CTE domains for PINK1 activation. (a) α-NTE and CTE PD-associated mutants lead to reduced protein stabilization, and loss of autophosphorylation and hPINK1 activation. Stably expressing PINK1-3FLAG WT, KI (D384A), empty vector (FLAG-emp), α-NTE mutants (C125G, Q126P), kinase domain mutants (A168P, E240K, G309D, G409V) and CTE domain mutants (L539F, ins534Q) cell lines were generated in PINK1-knockout Flp-In TRex-HeLa cells. PINK1-3FLAG expression was induced by 24 h treatment with 0.2 uM doxycycline, and mitochondrial depolarization induced by 3 h treatment with 10 µM A/O where indicated. Mitochondrial-enriched fractions were subjected to immunoblotting with α-PINK1 (in-house/DCP antibody), α-ubiquitin pS65 (CST), α-OPA1 (BD) and α-HSP-60 primary antibodies. n = 3. (b) Immunoblots were quantified for phospho-Ser65 Ub/HSP-60, PINK1/HSP-60 and pS228/HSP-60 using Image Studio software. Data are presented relative to WT hPINK as mean ± s.d. (n = 3). (c) Multiple sequence alignment of CTE region of PINK1 orthologues across species. Sequence alignment was performed with MUSCLE and annotated in Jalview. Mutated CTE residues for functional analysis are highlighted with arrow heads. (d) CTE mutants exhibit reduced stabilization, autophosphorylation and substrate phosphorylation. hPINK1 knockout HeLa cells transiently expressing hPINK1-3FLAG WT, KI or hPINK1 CTE mutants V528A (L503A in PhcPINK1), L532A (L507A in PhcPINK1) and L539A (L514A in PhcPINK1). Cells were stimulated ±10 μM CCCP for 6 h. Membrane fractions were isolated and solubilized in 1% Triton X-100 lysis buffer Lysates that were resolved by SDS-PAGE. Proteins were transferred to nitrocellulose membranes probed using the antibodies indicated. n = 2. (e) CTE triple mutant is inactive against recombinant substrates in vitro. Flp-In HEK293 PINK1 KO cells stably expressing WT or KI (D384A) or dimerization triple mutant (V528A/L532A/L539A) PINK1 were treated ±10 µM CCCP for 3 h and subjected to sub-cellular fraction in a CFAB. Three micrograms of non-solubilized membrane-enriched fraction (MeF) were incubated with tetraubiquitin (K63 linked) in CFAB supplemented with 2 mM (unlabelled) ATP, 5 mM MgCl2, 2 mM DTT and 0.75% Glycerol for 20 min. Samples were resolved via SDS-PAGE and blotted for the indicated antibodies. n = 2.