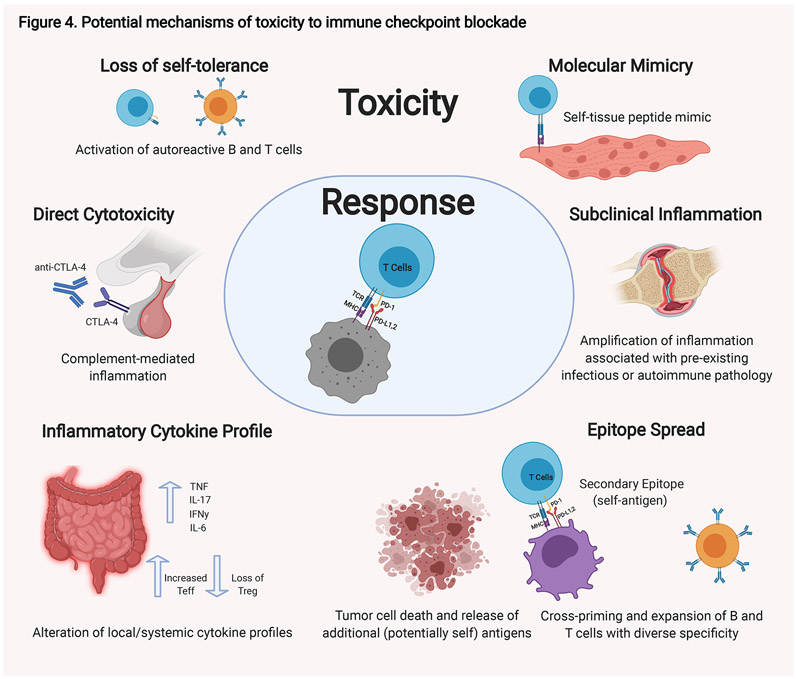
Figure 4. Potential mechanisms of toxicity to immune checkpoint blockade.
There are a number of possible mechanisms that have been proposed that contribute to the toxicities observed in some patients in response to immune checkpoint blockade. These possibilities are not mutually exclusive, and different mechanisms likely exist for different immune-related toxicities. Autoreactive T and B cells are thought to be key moieties in these processes. Autoreactive T cells could be generated de novo. These T cells are activated by professional APCs at the tumor site and reactive to tumor-specific antigens; however, they may coincidentally be reactive to peptides found on normal tissue that mimic the tumor-specific antigens. Alternatively, pre-existing autoreactive T and B cells that have escaped self-tolerance which were quiescent could be activated when self-peptides are presented through epitope spread by antigen presenting cells (APCs) at the tumor site. Immune-checkpoint blockade can result in alterations in the systemic immunity including changes in cytokine profiles. Changes in the cytokine profile within a given tissue can tip the existing balance towards inflammation. Alternative mechanisms also likely exist. For hypopituitarism, direct antibody-mediated cytotoxicity to CTLA-4 normally expressed on the pituitary gland is thought to play a role. Finally, amplification for pre-existing inflammatory or autoimmune pathologies are also possible. TNF, tumor necrosis factor; IFN, interferon; Teff, effector T cells; Treg, regulatory T cells.