Abstract
Hereditary haemoglobinopathies are common disorders in Oman. The most common haematological disorder among Omani population is sickle cell disease (SCD). The spleen is one of the organs that is affected early in the first decade of life in SCD patients. Splenectomy has shown a high success rate in improving the quality of life in SCD patients, through eliminating acute splenic sequestration crises, thus reducing the need for hospital admission and transfusion requirements. One of the rare complications of splenectomy is porto-splenic vein thrombosis. Multiple factors are responsible for this complication including: thermal and mechanical injury during ligation of splenic hilum, sudden increase in the platelet count and large spleen size. We report a rare case of extensive porto-splenic vein thrombosis that responded to early initiation of anticoagulation with resolution of the thrombosis and recanalisation.
Keywords: haematology (incl blood transfusion), paediatric surgery
Background
Hereditary haemoglobinopathies are common disorders in Oman. The most common haematological disorder among Omani population is sickle cell disease (SCD). There are around 3000 cases of SCD in Oman and around 10% of the Omani population are carriers for the sickle cell gene.1 There is no known cure for the disease since it is a genetic disorder except bone marrow transplant.2 3 There are recent studies of gene therapy to cure SCD, those trials are still being investigated.3
The spleen is one of the organs that is affected early in the first decade of life in SCD patients. The spleen commonly enlarges in early age mainly due to chronic haemolysis as well as blood sequestration. However, it gets atrophied later in life because of recurrent vaso-occlusion and infarctions.4 Acute splenic sequestration crises carry a high mortality rate reaching 15%–44% deaths during the first 5 years of life.5
Splenectomy has shown a high success rate in improving the quality of life in SCD patients; that is by eliminating the acute splenic sequestration crises, the need for hospital admission and transfusion requirements is reduced.6 The indications for splenectomy in SCD include hypersplenism, frequent acute splenic sequestration crises and splenic abscess.4 7 8
For many years, splenectomy has shown a high success and safety rate; however, complications may develop. In a study done in Oman on laparoscopic splenectomy between 2010 and 2015, Al Balushi et al reported a few postoperative complications including acute chest syndrome (15%) and pancreatitis (2%). None of the study patients developed other complications like overwhelming postsplenectomy infections, visceral injuries and portal or splenic vein thrombosis.8
We report a case of postsplenectomy portal and splenic vein thrombosis (PSVT). The course of the disease, surgical intervention, clinical presentation and management are discussed. We reviewed the possible pathophysiological mechanisms of PSVT and their treatment modalities.
Case presentation
A 7-year-old girl has SCD (HbS-S). She was diagnosed at the age of 1 year when she presented with severe pallor due to acute splenic sequestration crisis. On clinical examination, she was pale, with mild jaundice. Her spleen was 4 cm below the left costal margin. She was investigated at that time and her blood tests showed a Hb of 45 g/L, platelets of 66×109/L, LDH of 353 U/L, PT of 11.7 s, APTT of 32.3 s, TT of 15.2 s and fibrinogen of 2.6 g/L. A high-performance liquid chromatography showed 2.7% HbA2, 81.1% HbS, 15% HbF and 0% HbA. There was no HbSOman detected in the sample. She was labelled as sickle cell anaemia (HbS-S). She was admitted in the hospital, transfused packed red blood cells (PRBCs) and later discharged. After that she had recurrent splenic sequestration, initially every 4–6 months, then became more frequent. Her baseline Hb ranges between 70 g/L and 80 g/L. The patient was a candidate for splenectomy because of her large spleen and multiple episodes of splenic sequestration with severe exacerbation of her anaemia. She received pneumococcal 13 valent conjugate vaccine at 2, 4 and 6 months of age, as part of the national immunisation protocol and then two doses of pneumococcal polysaccharide 23 valent vaccine at the age of 2 and 5 years.
On the day of admission for surgery, she was pale with a spleen of 10 cm below the left costal margin, liver 3 cm below the right costal margin and the rest of examination was unremarkable. Her Hb was 64 g/L and platelet count was 188×109/L. She was transfused 400 mL of PRBCs and before surgery, her Hb raised up to 95 g/L. She underwent uneventful laparoscopic splenectomy. Dissection of the splenic hilum was done using ultracision. Pathology report of the spleen showed splenic tissue with hyperplastic follicles and massively dilated and congested sinuses, and scattered fibrotic nodule with hemosiderin-laden macrophages and calcium depositions (Gamna-Gandy bodies). The pools of RBCs within the sinuses show sickled morphology. There was no evidence of malignancy in the examined tissue. There was no evidence of splenic vein thrombosis.
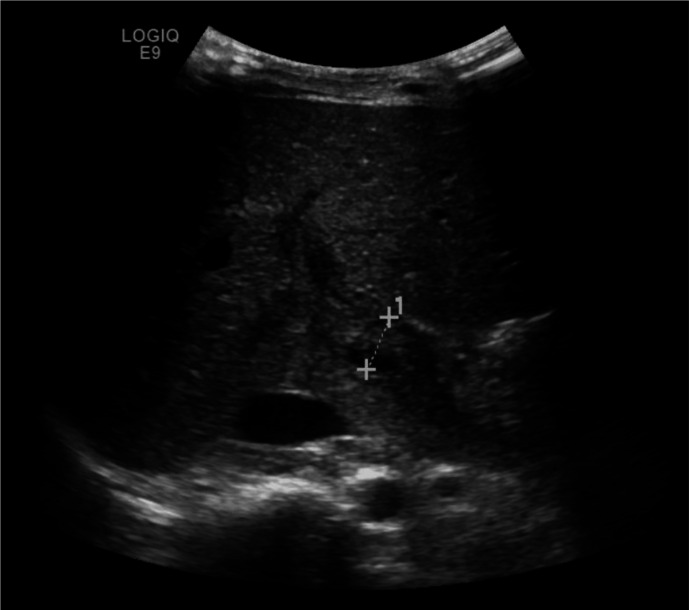
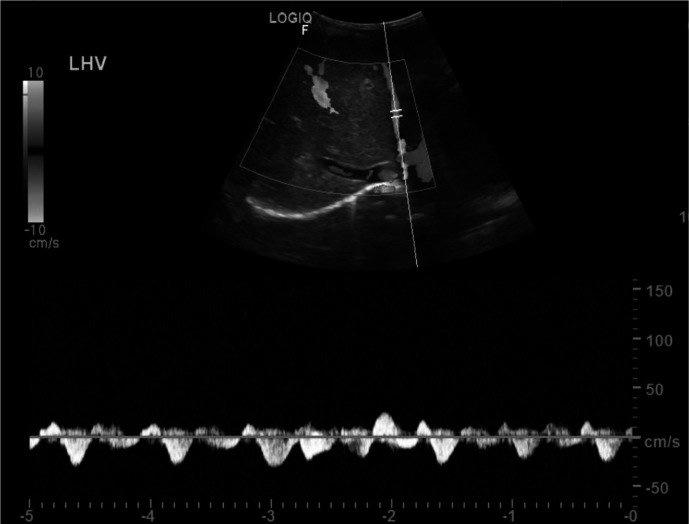
Postoperatively, she was doing well and discharged home 2 days after the surgery. Her Hb was 83 g/L, and platelet count was 242×109/L. Ten days after the surgery, the patient presented to the hospital complaining of severe abdominal and back pain of 3 days duration. On clinical examination, she was markedly pale with mild jaundice. Her abdomen was tense with tender hepatomegaly of 6 cm below the right costal margin. Rest of examination was unremarkable. She was admitted and started on intravenous fluids and analgesia. Initial laboratory investigations showed a Hb of 53 g/L and platelet count 584×109/L. An abdominal ultrasound (US) showed portal vein and superior mesenteric vein thrombosis (figure 1). A CT scan confirmed that thrombosis was involving the superior mesenteric, splenic and portal veins with patent hepatic veins and intrahepatic segment of Inferior vena cava (IVC). There was mesenteric congestion oedema and minimal fluid. No CT evidence of bowel ischaemia. The patient was admitted in paediatric haematology ward and managed with top-up and exchange blood transfusion to reduce HbS to below 30%. Low molecular weight heparin (LMWH) was started at therapeutic dose of 0.7 mg/kg two times per day for 14 days. Clinically, she has improved with resolution of abdominal pain, liver was normal in size and not tender. She was alert and active and feeding well. A US was repeated on the 11th day of admission and showed partial recanalisation of the previously thrombosed portal and superior mesenteric veins with evidence of cavernous transformation (figure 2). Patient was discharged home on once daily prophylactic dose of LMWH of 0.7 mg/kg for 6 weeks and her anti-Xa was kept between 0.6 U/mL and 1 U/mL. On follow-up in the out-patient department (OPD) after 2 months from discharge, the patient looked healthy with no complaints and her physical examination was unremarkable. Her Hb was 76 g/L, and platelet count was 435×109/L, lupus anticoagulant was not detected and thrombophilia workup including factor V Leiden, protein C, protein S and antithrombin deficiency was negative. Repeat US showed complete resolution of the thrombosis with complete recanalisation of the veins.
Figure 1.
Ultrasonography of the abdomen showing main portal vein with porta hepatis vein thrombosis.
Figure 2.
Ultrasonography of the abdomen showing traces of colour flow in the left portal vein indicating revascularisation of the previously thrombosed vein.
Discussion
This is the first reported case of thrombosis postsplenectomy in Oman. This is a rare complication with few reported cases in the literature. Patients usually present with non-specific symptoms including abdominal pain, nausea and vomiting, which make the diagnosis difficult. In children, this complication is more common in patients with hereditary stomatocytosis.9
In similarity to our patient, Miniati et al reported a 16-year-old girl with immune thrombocytopenia (ITP) who had laparoscopic splenectomy. During the surgery, hilum was dissected with GIA stapler, blood loss was 20 mL and the duration was 106 min. She presented to the hospital with abdominal pain 20 days after the surgery. She was found to have extensive thrombosis of the portal vein, splenic vein and superior mesenteric vein. She received anticoagulation and improved clinically. A US after 6 months showed recanalisation but incomplete resolution of the thrombus unlike in our case.10 Preoperatively our patient received top-up transfusion to raise the Hb to above 90 g/L. We stopped preparing our SCD patients with exchange transfusion before surgeries including major ones. We have an interesting clinical trial published in ‘Transfusion’ highlighting the practice in our institution.11 We do top-up transfusion only if Hb is below 90 g/L.
The pathophysiology behind portal system venous thrombosis is not well established. Multiple factors have been suspected to contribute to this complication including thermal and mechanical injury during ligation of splenic hilum, sudden increase in the platelet count and large spleen size.12
In our patient, ultracision used might induce thermal injury to the hilum which might have contributed to the thrombus formation. However, the same device has been used in our previous 150 patients who underwent splenectomy, but none of them developed thrombosis.
The spleen has an important role in clearing up prothrombotic factors. This function is lost after splenectomy. Together with thrombocytosis, these are considerable factors contributing to thrombosis.9 Our patient had a significant increase in platelet counts which was 188×109/L preoperatively and 584×109/L postoperatively. This sharp bouncing of the platelet count after surgery might have contributed to thrombosis. However, it is worth mentioning that a previous study on 39 patients found no significant association between platelet count and PSVT.13
Weight of the spleen has been found to have a significant correlation with the incidence of PSVT.13–15 A possible pathophysiology is that large spleen has a larger splenic vein diameter, so after splenectomy the blood flow through the splenic vein slows significantly which might contribute to thrombus formation.16 In our case, the spleen weight was 1.5 kg which is quite large. No specific weight mentioned in the literature that can be specified as a risk factor for PSVT. The diameter of the splenic vein preoperatively is a considerable risk factor for PSVT. It was mentioned in the literature that a diameter more than 10 mm is a sensitive and specific risk factor for PSVT. Unfortunately, we do not have a baseline CT scan for our patient.
Of interest that in our patient, the response to LMWH was very fast with almost complete recanalisation after 11 days of therapy. In addition, the clinical symptoms resolved quickly within a few days of LMWH therapy initiation. In previous case reports, the response to the LMWH was delayed up to 6 months or even more.10 This fast recovery in our patient can be explained by the early presentation to the hospital with first sign of abdominal pain, early detection of the thrombosis by US Doppler and CT in the same day of presentation and the immediate initiation of specific anticoagulation therapy. Of note that our patient has no other possible risk factors as thrombophilic disorders and lupus anticoagulant, however, taking into consideration the enormous spleen size and the expected postsplenectomy thrombocytosis, short-term LMWH might have been introduced prophylactically after surgery.
Although there is no specific treatment protocol for PSVT after splenectomy, early recognition of the problem and initiation of treatment might avoid fatal outcomes.17 18 Treatment modalities are variable and include systemic anticoagulation or thrombolysis and catheter directed thrombolysis or thrombectomy.17 Catheter directed thrombolysis or thrombectomy is indicated in cases of established ischaemia or tissue necrosis,15 otherwise early initiation of anticoagulation promotes recanalisation and has success rate up to 35%.19
Patient’s perspective.
I am the mother of the patient. Although initially I was shocked with the severe abdominal pain postsurgery, yet I was happy with the speed of reaching the correct diagnosis and the fast recovery of my daughter.
Learning points.
Portal and splenic vein thrombosis (PSVT) is a very rare complication of splenectomy.
It has multifactorial risk factors including: large spleen size, large splenic vein diameter, thermal injury and thrombophilia disorders.
Laparoscopic splenectomy can still be done even with very large size of the spleen but with more strict follow-up for possible complications including PSVT; discharge on day 3 was probably premature.
Early initiation of anticoagulation in these cases might be life-saving and results in fast recovery.
Footnotes
Contributors: TAB wrote down the case report and the discussion part with help of SAS who managed the patient in the first admission. YW and ZAB reviewed the case report and the analysis part.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained from parent(s)/guardian(s).
References
- 1.Rajab A, Al Rashdi I, Al Salmi Q. Genetic services and testing in the sultanate of Oman. Sultanate of Oman steps into modern genetics. J Community Genet 2013;4:391–7. 10.1007/s12687-013-0153-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jose J, Elsadek RA, Jimmy B, et al. Hydroxyurea: pattern of use, patient adherence, and safety profile in patients with sickle cell disease in Oman. Oman Med J 2019;34:327–35. 10.5001/omj.2019.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demirci S, Uchida N, Tisdale JF. Gene therapy for sickle cell disease: an update. Cytotherapy 2018;20:899–910. 10.1016/j.jcyt.2018.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Salem AH. The role of splenectomy in patients with sickle cell disease. Ann Saudi Med 1997;17:316–20 https://www.annsaudimed.net/doi/ 10.5144/0256-4947.1997.316 [DOI] [PubMed] [Google Scholar]
- 5.Topley JM, Rogers DW, Stevens MC, et al. Acute splenic sequestration and hypersplenism in the first five years in homozygous sickle cell disease. Arch Dis Child 1981;56:765–9. 10.1136/adc.56.10.765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al Salem A. Indications and complications of splenectomy for children with sickle cell disease, 2006. Available: https://www.jpedsurg.org/article/S0022-3468(06)00472-6/fulltext [Accessed 08 Apr 2021]. [DOI] [PubMed]
- 7.El-Hazmi MAF, Al-Hazmi AM, Warsy AS. Sickle cell disease in Middle East Arab countries. Indian J Med Res 2011;134:597–610. 10.4103/0971-5916.90984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Balushi ZN, Bhatti KM, Ehsan MT, et al. Laparoscopic splenectomy alone for sickle cell disease: account of 50 paediatric cases. Sultan Qaboos Univ Med J 2016;16:e482–6. 10.18295/squmj.2016.16.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krauth M-T, Lechner K, Neugebauer EAM, et al. The postoperative splenic/portal vein thrombosis after splenectomy and its prevention--an unresolved issue. Haematologica 2008;93:1227–32 https://haematologica.org/article/view/4964 10.3324/haematol.12682 [DOI] [PubMed] [Google Scholar]
- 10.Miniati DN, Padidar AM, Kee ST, et al. Portal vein thrombosis after laparoscopic splenectomy: an ongoing clinical challenge. JSLS 2005;9:335–8. [PMC free article] [PubMed] [Google Scholar]
- 11.Elshinawy M, Al Marhoobi N, Al Abri R, et al. Preoperative transfusion versus no transfusion policy in sickle cell disease patients: a randomized trial. Transfusion 2020;60 Suppl 1:S22–7. 10.1111/trf.15684 [DOI] [PubMed] [Google Scholar]
- 12.Sun L, Zhou H, Gu L, et al. Effects of surgical procedures on the occurrence and development of postoperative portal vein thrombosis in patients with cirrhosis complicated by portal hypertension. Int J Surg 2015;16:31–5. 10.1016/j.ijsu.2015.02.005 [DOI] [PubMed] [Google Scholar]
- 13.Portal or splenic vein thrombosis after splenectomy for immune cytopenia: a retrospective cohort study, 2015. Available: https://ashpublications.org/blood/article/126/23/3483/90804/Portal-or-Splenic-Vein-Thrombosis-after [Accessed 17 May 2021].
- 14.Krauth M-T, Lechner K, Neugebauer EAM, et al. The postoperative splenic/portal vein thrombosis after splenectomy and its prevention--an unresolved issue. Haematologica 2008;93:1227–32. 10.3324/haematol.12682 [DOI] [PubMed] [Google Scholar]
- 15.Ikeda M, Sekimoto M, Takiguchi S, et al. Total splenic vein thrombosis after laparoscopic splenectomy: a possible candidate for treatment. Am J Surg 2007;193:21–5 https://www.americanjournalofsurgery.com/article/S0002-9610(06)00690-8/fulltext 10.1016/j.amjsurg.2006.06.036 [DOI] [PubMed] [Google Scholar]
- 16.Rattner DW, Ellman L, Warshaw AL. Portal vein thrombosis after elective splenectomy. An underappreciated, potentially lethal syndrome. Arch Surg 1993;128:565–70. 10.1001/archsurg.1993.01420170101015 [DOI] [PubMed] [Google Scholar]
- 17.Condat B, Pessione F, Helene Denninger M, et al. Recent portal or mesenteric venous thrombosis: increased recognition and frequent recanalization on anticoagulant therapy. Hepatology 2000;32:466–70. 10.1053/jhep.2000.16597 [DOI] [PubMed] [Google Scholar]
- 18.Turnes J. Portal hypertension–related complications after acute portal vein thrombosis: impact of early anticoagulation, 2008. Available: https://www.cghjournal.org/article/S1542-3565(08)00798-2/fulltext [Accessed 11 Apr 2021]. [DOI] [PubMed]
- 19.Condat B, Pessione F, Helene Denninger M, et al. Recent portal or mesenteric venous thrombosis: increased recognition and frequent recanalization on anticoagulant therapy. Hepatology 2000;32:466–70. 10.1053/jhep.2000.16597 [DOI] [PubMed] [Google Scholar]