Structured Abstract
BACKGROUND:
Historical accounts linking cancer and microbes date as early as four millennia ago. Post establishment of the germ theory of infectious diseases, clinical research of microbial influences on cancer began in 1868, when William Busch reported spontaneous tumor regressions in patients with Streptococcus pyogenes infections. Over the next century, the role of bacteria in carcinogenesis and cancer therapy was discounted due to poor reproducibility, erroneous microbiological claims, and severe toxicity in patients. However, these provided some of the first crude demonstrations of cancer immunotherapy. Contemporaneously, the viral theory of cancer began to flourish, spurred by the 1911 discovery of Rous Sarcoma Virus (RSV), which transformed benign tissue into malignant tumors in domestic fowl. The subsequent decades-long search to find a virus behind every human cancer ultimately failed, and many cancers have been fundamentally linked to somatic mutations. Now the field is encountering intriguing claims of the importance of microbes, including bacteria and fungi, in cancer and cancer therapy. This Review critically evaluates the evidence for these claims in light of modern cancer biology and immunology, and delineates the roles of microbes in cancer by examining recent advances in proposed mechanisms, diagnostics, endogenous modulation approaches, and exogenous therapeutic strategies.
ADVANCES:
Few microbes directly cause cancer, but many seem complicit in its growth, often acting through the host’s immune system; conversely, several have immunostimulatory properties. Mechanistic analyses of gut microbiota-immune system interactions have demonstrated powerful effects on innate and adaptive immunity by modulating primary and secondary lymphoid tissue activities against cancer and tumor immunosurveillance. Many of these pathways invoke Toll-like receptor (TLR)-initiated cytokine signaling, but microbial metabolic effects in dietary energy harvest and short-chain fatty acid production, and antigenic mimicry with cancer cells, are also important. In preclinical models, microbial metabolites also regulate phenotypes of tumor somatic mutations and modulate immune checkpoint inhibitor efficacy.
Emerging evidence also suggests the existence and functional activity of intratumoral bacteria, with overlapping immunohistochemistry, immunofluorescence, electron microscopy, and sequencing data on them in ~10 cancer types. Preliminary studies also suggest that fungi and bacteriophages contribute to gastrointestinal cancers. However, the estimated cellular abundances of intratumoral microbes is low relative to cancer cells, and knowledge of their functional repertoire and potency remains limited. Further validation of their prevalence and impact is needed in diverse cohorts and therapeutic contexts.
The immunomodulatory effects of host microbiota have reinvigorated efforts to change their composition as a form of immunotherapy. Despite extensive preclinical evidence, translation of microbiota modulation approaches into humans has yet to broadly materialize into commercialized therapies. Synthetic biology approaches are also gaining traction, however, with engineered bacterial cancer therapies in preclinical and clinical trial settings.
OUTLOOK:
A better understanding of the roles of microbes in cancer has the opportunity to improve each stage of the cancer care cycle, but major challenges must be surmounted. Concerted efforts to characterize cancer-associated microbiota among tumor, stool, and blood samples with gold-standard contamination controls would tremendously aid this progress. This would be analogous to The Cancer Genome Atlas (TCGA)’s and International Cancer Genome Consortium (ICGC)’s roles in characterizing the cancer somatic mutation landscape. Large-scale clinical trials are currently testing the efficacy of microbiota modulation approaches, ranging from dietary modifications to intratumorally-injected, engineered bacteria. These bacterial cancer therapies, if safe and effective, could tremendously expand the cancer therapy armamentarium. Altogether, integrating the host-centric and microbial viewpoints of cancer may improve patient outcomes while providing a nuanced understanding of cancer-host-microbial evolution.
Graphical Abstract
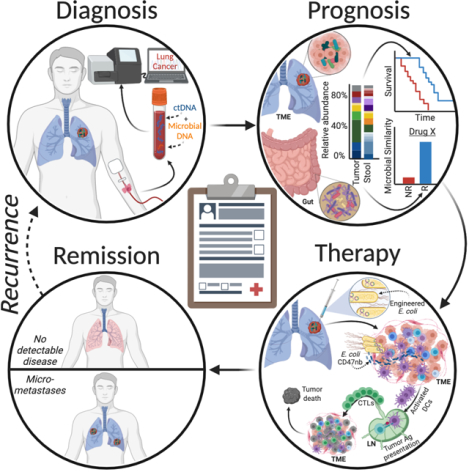
Opportunities for microbes to impact cancer care. Diagnosis: Cancer-specific, blood-borne microbial DNA may complement cell-free tumor DNA (ctDNA). Prognosis: Gut and intratumoral microbiota may stratify patient outcomes; (N)R=(non)responder; TME=tumor microenvironment. Therapy: Intratumor injection of CD47 nanobody (CD47nb)-producing E. coli may create systemic antitumor immunity by enhancing dendritic cell (DC) phagocytosis, lymph node (LN) antigen (Ag) presentation, and cytotoxic T lymphocyte (CTL) activity.
Abstract
Microbial roles in cancer formation, diagnosis, prognosis, and treatment have been disputed for centuries. Recent studies have provocatively claimed that bacteria, viruses, and/or fungi are pervasive among cancers, key actors in cancer immunotherapy, and engineerable to treat metastases. Despite these findings, the number of microbes known to directly cause carcinogenesis remains small. Critically evaluating and building frameworks for such evidence in light of modern cancer biology is an important task. In this Review, we delineate between causal and complicit roles of microbes in cancer and trace common themes of their influence through the host’s immune system, herein defined as the immuno-oncology-microbiome (IOM) axis. We further review evidence for intratumoral microbes and approaches that manipulate the host’s gut or tumor microbiome while projecting the next phase of experimental discovery.
The histories of cancer and human microbiota are intimately interwoven. Writings as early as 1550 BCE in the Ebers Papyrus, attributed to the Egyptian physician Imhotep (c 2600 BCE), suggest a crude treatment for tumors (swellings) involving application of a poultice to the site followed by an incision, causing an infection (1, 2). In the 13th century, Peregrine Laziosi described spontaneous regression of his septic, ulcerative tibial bone malignancy that would have required amputation (2), for which he was canonized in 1726. After establishment of the germ theory of infectious disease, Wilhelm Busch and Friedrich Fehleisen independently reported in the late 1800s that Streptococcus pyogenes infections were associated with spontaneous tumor regressions in several patients (3, 4). Shortly thereafter, William Coley started testing a highly contentious and sometimes lethal vaccine of live or heat-killed Streptococcus and Serratia species on terminal cancer patients, which was only later shown to yield >10-year disease-free survival in ~30% of them (60 of 210 total), representing the first intentional demonstration of immunotherapy (5). Contemporaneously, Thomas Glover and Virginia Livingston-Wheeler claimed, controversially, that bacteria were cultivable from tumors and that bacterial vaccines were effective against tumors, and suggested a universal bacterial origin of cancer (6, 7). These early treatment approaches and theories were fraught with error: Livingston-Wheeler’s bacterial “cause” of cancer, Progenitor cryptocides, turned out to be the skin commensal Staphylococcus epidermidis (a frequent contaminant), and Glover’s findings were not reproducible by researchers at the National Institutes of Health (7). With no mechanistic evidence, irreproducible results, and hazardous therapies, the bacterial theory of cancer was dismissed.
The viral theory of cancer gained traction after Peyton Rous’s 1911 discovery of a transmissible oncogenic virus in chickens (8). The subsequent decades-long search to find a virus behind every cancer linked Epstein-Barr, human papilloma, and hepatitis viruses to carcinogenesis (9) but failed to find a viral cause for most human cancers, and the theory was overtaken by the somatic mutation hypothesis.
Now, after decades of research thoughtfully characterizing the hallmarks of human cancer through somatic mutations and other host-centric perspectives (10, 11), the field is encountering nuanced claims that microbes may play a broad role in cancer diagnosis, pathogenesis, and treatment (12–26). This reappraisal stems from greater appreciation of the number of microbes that inhabit the human body (roughly equal to the number of human cells), their gene count that exceeds the human genome’s gene count by ~100-fold and enables diverse metabolic programming, and their effects on host immune system development and activity, including antitumor immunosurveillance (27–31). Although most proposed cancer-microbe relationships focus on gut microbiota (30, 32, 33), recent studies also contentiously suggest the existence, metabolic activity, and functional importance of intratumoral microbiota using a combination of imaging, sequencing, and cultivation techniques, and genetically-engineered and germ-free mouse models (12–14, 18–20, 23, 34). These studies raise many questions about microbes and cancer. How should microbes be viewed in light of known host-centric cancer characteristics? To what extent are microbes causal agents, complicit actors, or passive bystanders? If intratumoral microbes exist, do they have therapeutic implications? What role do microbes play in patient management? With these questions in mind, this Review aims to critically evaluate the known roles of microbes in cancer, and to outline the next steps for evaluating their clinical utility.
Overview of the cancer microbiome
Of the estimated ~1012 distinct microbial species on earth (35), just 11 are labeled human carcinogens, or “oncomicrobes,” by the International Association for Cancer Registries (IACR) (36). These oncomicrobes cause an estimated 2.2 million cases per year (~13% of global cancer cases), and their epidemiology, molecular mechanisms, and clinical studies have been extensively reviewed (36). Strong experimental evidence suggests that additional microbes initiate cancer through genotoxin-mediated mutagenesis; in particular, colibactin (a DNA alkylator), cytolethal distending toxin (CDT; direct DNAse activity), and Bacteroides fragilis toxin (Bft; ROS producer) cause mutational signatures found in colorectal, head and neck, and urinary tract cancers (22, 37–41). Experimental evidence also implicates several microbes with virulence factors that amplify tumorigenesis via E-cadherin/Wnt/β-Catenin signaling, including FadA from Fusobacterium nucleatum and AvrA from several Salmonella strains (42, 43). A few dozen microbial species can thus directly cause cancer, based on current epidemiological and experimental evidence.
Increasing evidence suggests an important additional category of “complicit” microbes and microbial functions that promote carcinogenesis but are insufficient to cause cancer (18, 20, 25, 38, 44–47). This category encapsulates many immunomodulatory functions of microbiota and their bioactive metabolites in tumor development, and may be linked to the immune system’s role in solid tumorigenesis; the immune system rarely initiates the incipient lesion but can facilitate progression through tumor-stroma feedback loops, inflammation, or dysfunctional immunosurveillance (11). One example is that common p53 mutations are only carcinogenic in the presence of microbially-produced gallic acid and protective otherwise in the gut, both in vivo and in organoids, suggesting a microbiome-functional genomic interaction (44). A second is microbially-produced secondary bile acids, which reduce hepatic sinusoidal CXCL16 expression (the sole ligand for CXCR6) and prevent CXCR6+ natural killer T (NKT) cell aggregation and liver cancer immunosurveillance — this carcinogenic effect is eliminated by vancomycin treatment (45). A third comes from the inability of Kras mutation and p53 loss to produce lung cancer in germ-free or antibiotic-treated mice: commensal lung microbiota promote expansion and activation of γδ T cells, which drives tumor-promoting inflammation via local IL-17 and IL-23 release (18). These examples illustrate how microbes or microbial functions can be complicit in cancer rather than directly causal.
In contrast to the few bona fide oncomicrobes, the many “complicit” microbes and their functions are broad and under-studied. Complicit microbes require mediators to promote tumor development, but modulate tumor progression and therapeutic efficacy locally or from a distance. Complicit microbes are also least understood, requiring comprehension and integration of host and microbial biology, so we emphasize them in this Review. Together with known causal mechanisms, the diversity of these “complicit” mechanisms and their relationships to host-centric cancer hallmarks (10, 11) are notable (Fig. 1), but they will require more rigorous experimentation and cross-cohort validation to establish clinical prevalence and utility.
Fig. 1. Examples by which microbial mechanisms intersect with key cancer pathways (10, 11).
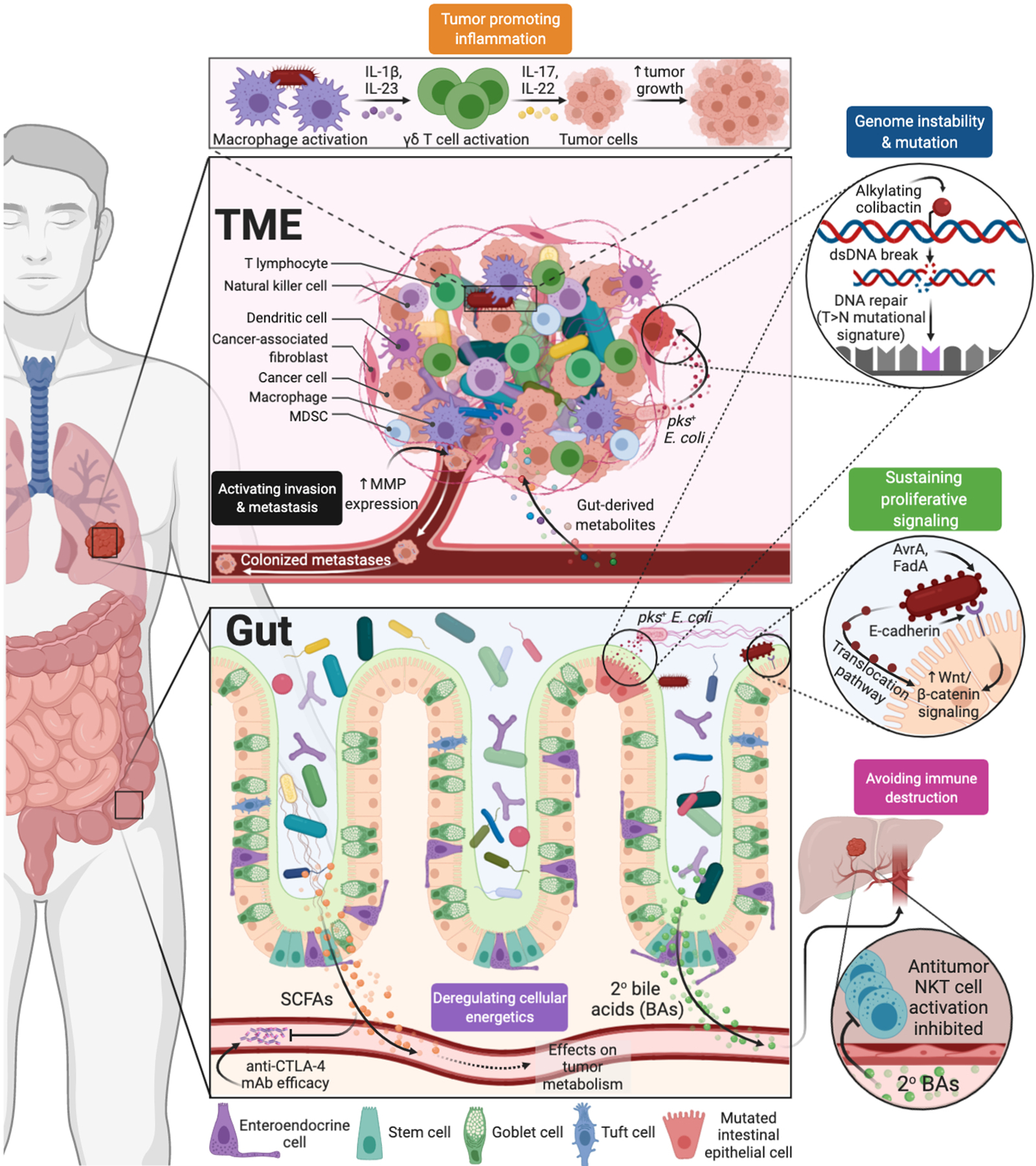
Microbiota-derived metabolites, genotoxins, and antigens influence host antitumor immunity, inflammation, energetics, cellular signaling, and metastasis. Abbreviations: MMP=matrix metalloproteinases; SCFAs=short-chain fatty acids; mAb=monoclonal antibody.
Understanding the distribution of microbes across the body is important for understanding their relationships to cancer. Approximately 4×1013 microbial cells spanning ~3×103 species inhabit the human body: ~97% of these cells are bacteria in the colon, ~2–3% extra-colonic bacteria (proximal gut, skin, lungs, etc.), and ~0.1–1% archaea and eukarya (including fungi) (27, 48). Human-infecting virus and phage counts and diversity may be greater (49). The high density of colonic bacteria is thought to drive the majority of known microbial immunomodulatory effects in the mammalian intestinal tract, the largest immune organ in the body (50), but organ-specific commensals may exert their own overriding influence (18, 46). Intratumorally, Nejman et al. used quantitative PCR (qPCR) of 16S rRNA to estimate the number of bacteria relative to 40 nanograms of DNA in melanoma, lung, ovarian, glioblastoma, pancreatic, bone, and breast cancer tissue sections (12). Assuming tissue homogeneity and 8 picograms of DNA per cancer cell (based on 2.36 average tumor ploidy from the Pan-Cancer Analysis of Whole Genomes project) (51), the Nejman et al. data suggest an average pan-cancer percent bacteria composition at 0.68% bacterial (bootstrapped 95% CI of mean:[0.52%, 0.87%], 1000 iterations), with individual tumors ranging from no bacteria to nearly 70% bacterial by cell count (12). Applying this percent bacterial composition to three dimensional and planar contexts equates to ~105-106 bacteria per palpable 1 cm3 tumor (52) or ~34 bacteria/mm2 (assuming 5000 cells/mm2 (53)), the latter of which is comparable to the average PD1+ T lymphocyte tumor core density of ~21 cells/mm2 from a recent pan-cancer cohort (54). Importantly, these bacterial composition estimates remain to be confirmed in other cohorts and cancer types and validated with orthogonal methods. Furthermore, which of these microbial taxa and functions can affect the host despite their low abundances remains unknown, as does the proportion that are merely passengers in a nutrient-rich and immunosuppressed space.
Mechanisms and interactions between the gut and tumor microbiome
Gut microbiota can regulate many functions of the tumor-bearing meta-organism, typically through immunomodulation, and putative intratumoral microbes may also be important (31, 55). Known microbial mechanisms can manipulate non-hematopoietic and hematopoietic components of the gut epithelial barrier, modulate primary and secondary lymphoid organ activities, and regulate immune tone of the tumor microenvironment (TME). We define these immune-mediated interactions and collective feedback loops as the immuno-oncology-microbiome (IOM) axis (Fig. 2). Gut-TME crosstalk, especially in non-gastrointestinal cancers, remains a key area of discovery.
Fig. 2. Defining the immuno-oncology-microbiome (IOM) axis.
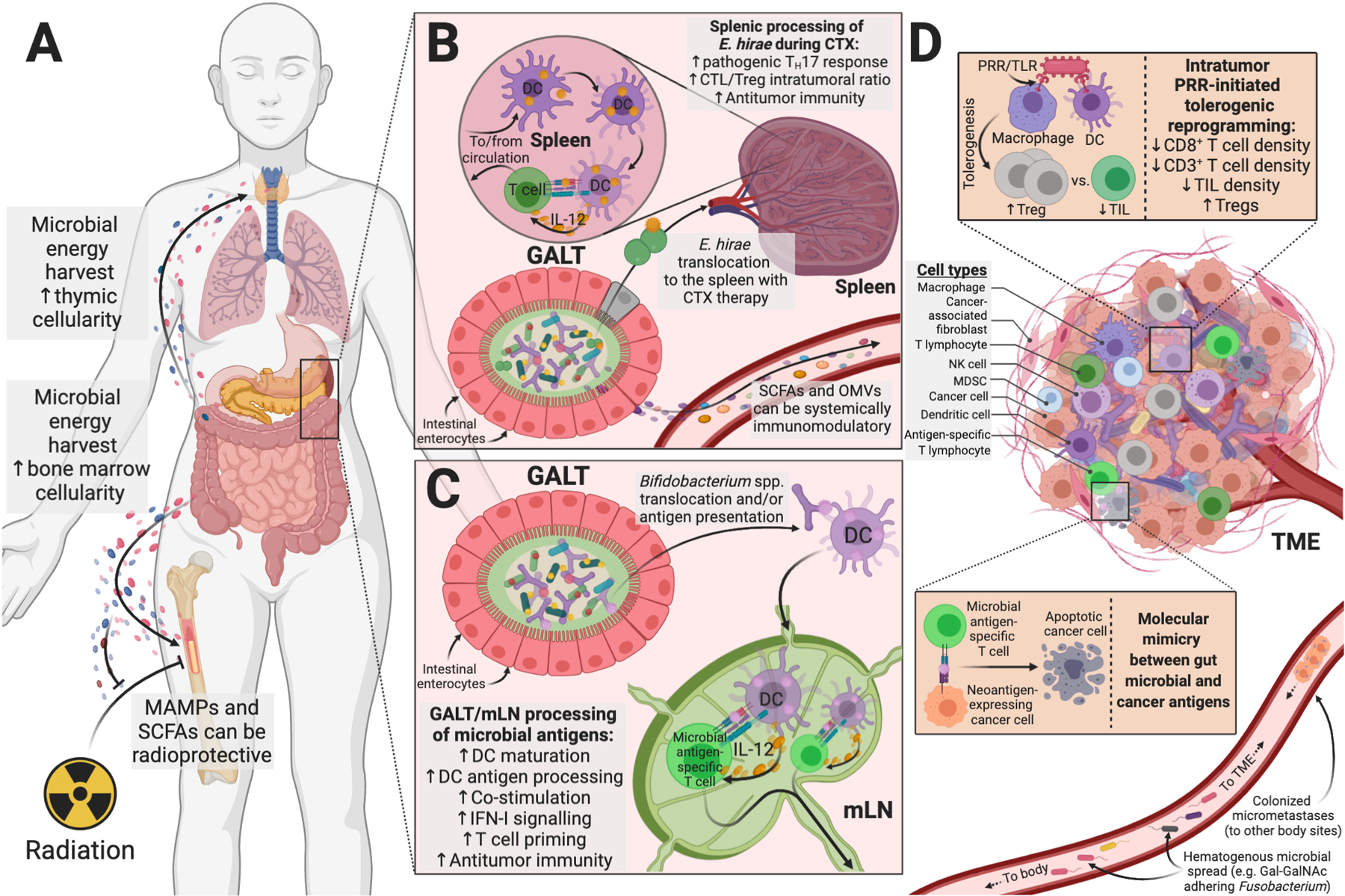
Gut and TME microbiota regulate host metabolism and immunity, which ultimately influence antitumor immunity. (A) Gut microbial metabolites and byproducts influence host lympho- and myelopoiesis, including during allogeneic HSCT and radiotherapy (59, 63). (B) Cyclophosphamide (CTX)-derived gut epithelial damage enables E. hirae translocation and antitumor immunity (68, 69). (C) Gut translocation of Bifidobacterium species or its antigens can increase IFN-I signaling and antitumor immunity (73, 76). (D) Microbes within the tumor microenvironment (TME) can be either immunosuppressive (often PRR-mediated) or immunogenic, including shaping response to immunotherapy (12, 23). Cancer (neo)antigens may share epitopes with microbes through molecular mimicry (73, 83). Microbial hematogenous spread (117, 171, 172) or colonized micrometastases (19) may complete this feedback loop that originated in the gut. Abbreviations: MAMPs=microbe-associated molecular pattern; SCFAs=short-chain fatty acids; GALT=gut-associated lymphoid tissue; mLN=mesenteric lymph node; DC=dendritic cell; OMVs=bacterial outer membrane vesicles; NK=natural killer cell; PRR=pattern recognition receptor; TIL=tumor-infiltrating lymphocytes.
The effects of gut microbiota on primary lymphoid organs
Following allogeneic hematopoietic stem cell transplantation (HSCT), robust immune reconstitution governs both relapse and transplant-related patient mortality (56, 57). A recent multi-center, multinational clinical trial demonstrated that higher diversity of intestinal microbiota is significantly associated with lower patient mortality following allogeneic HSCT (58). Moreover, in an allogeneic HSCT trial that co-analyzed daily changes in patient differential blood counts with >10,000 longitudinal fecal samples, immune-reconstitution dynamics were closely linked to gut microbiota composition (59). Links between gut microbiota, nutrition, post-transplant bone marrow (BM) and thymic cellularity, and lympho- and myelopoiesis, have also been demonstrated in mouse models (57). Gut microbe depletion impairs systemic infection clearance after BM transplant, and sensitizes mice to semi-lethal doses of radiation. Microbiota-derived compounds can protect against irradiation-induced hematopoietic injury (60–62) through producing propionate and tryptophan metabolites (63) or by releasing MAMPs known to maintain BM-derived myeloid cells and neutrophil function (64, 65). This effect may be explained in part by the delivery of endogenous ligands for RIG-I (such as 3pRNA and RNA derived from viruses, phages, or bacteria) that can induce protective type I interferon (IFN-I) signaling in enterocytes and intestinal barrier repair (66). Post-transplant lymphopoiesis also depends on energy harvest from the diet and potentially on genera whose genomic repertoires encode carbohydrate-active enzymes (57).
The effects of gut microbiota on adaptive immunity
The gut microbiota has broad effects contributing to host immune tone at steady state and during tumorigenesis (30, 67). Anti-cancer therapies have demonstrated strong links between distinct commensals and protective antitumor T cell responses: (i) Cyclophosphamide enables Enterococcus hirae to translocate and stimulate pathogenic TH17 responses and IFN-producing CD8+ T cell effectors that check tumor growth in sarcoma and lung adenocarcinoma models (68, 69); (ii) in some patients with melanoma, CTLA-4 blockade allows fecal relative enrichment of B. thetaiotaomicron and B. fragilis that mediates TLR4- and IL-12-dependent TH1 responses and therapeutic efficacy (70); (iii) PD-(L)1 inhibition leads to T cell priming against melanoma and is more effective when hosts harbor Bifidobacteria species in their microbiome (21, 71); (iv) adoptive T cell transfer efficacy against melanoma after total body irradiation depends upon the composition of the microbiota, the translocation of gut bacteria, and host TLR4 signaling (72–74); (v) oxaliplatin-induced cell death of ileal enterocytes inversely governs the immunogenic Erysipelotrichaceae and tolerogenic Fusobacteriaceae proportions in the ileum, dictating the balance between antitumor follicular T helper cells (TFH) and deleterious TH17 responses in colon cancer (75).
In most of these models, dendritic cells (DC) from the gut-associated lymphoid tissue (GALT), spleen, or tumor draining lymph node (LN) sense various commensals (Bifidobacterium spp., B. fragilis, A. muciniphila, B. rodentum, Bacteroidales S24–7), catalyzing immune responses via IFN-I and IL-12-mediated pathways (17, 70–72, 75–77). Apart from providing DC adjuvants, the gut microbiota represent an antigen source that can elicit commensal-specific T cell responses systemically (55, 78). In the context of homologous self-antigens, these commensal-specific immune responses can be deleterious or protective for the host, depending on the involved peptide(s). For instance, Gil-Cruz and colleagues demonstrated how homology between B. thetaiotaomicron-derived β-galactosidase and host cardiac myosin heavy chain 6 could drive lethal autoimmune inflammatory cardiomyopathy (79); conversely, Nanjundappa and others reported how cross-reactivity between Bacteroides species-derived integrase and host islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP) could hijack autoreactive CD8+ T cells to instead suppress colitis (80). Recent studies have further expanded this cross-reactive homolog list to include exogenous dietary antigens, notably between gliadin epitopes and gut Pseudomonas fluorescens-derived succinyl-glutamate desuccinylase in the context of HLA-DQ2.5-mediated celiac disease (81). Molecular mimicry between cancer and microbial antigens has also been hinted (82) and recently studied in-depth (83). H-2Kb-restricted T cell immune responses against a phage that infects distinct strains of enterococci (E. hirae) cross-reacted with an oncogenic driver (PSMB4). Oral administration of E. hirae strains containing this phage then boosted phage-specific T cell responses effective against extra-intestinal tumors overexpressing PSMB4 during therapy with cyclophosphamide or anti-PD1 antibodies (83). Similarly, T cells targeting an epitope, SVYRYYGL (SVY), expressed in the commensal bacterium Bifidobacterium breve, cross-reacted with a model neoantigen, SIYRYYGL (SIY), expressed by mouse melanoma B16-SIY (73). Moreover, some human T cells specific for naturally processed melanoma epitopes were found to recognize microbial peptides (83), suggesting clinical significance. However, mechanisms outside of molecular mimicry that boost antitumor immunity must also exist. For example, Tanoue and colleagues identified an 11-bacteria cocktail that increased tumor antigen-specific CD8+ IFN-γ+ T cells in the context of immune checkpoint blockade that were not cross-reactive with microbial antigens and did not originate from the colon (84).
Gut-derived metabolites can also modulate immune responses. Radiotherapy of tumor lesions was more effective when vancomycin eliminated Clostridiales-derived immunosuppressive metabolites (butyrate and propionate), putatively by increasing DC antigen presentation and concomitant CD8+ T cell priming (72); conversely, gut microbial-derived propionate and tryptophan pathway metabolites (1H-indole-3-carboxaldehyde, kynurenic acid) were shown to provide long-term radioprotection in vivo (63). High blood butyrate and propionate levels were also associated with resistance to CTLA-4 blockade in mice and melanoma patients, with concomitantly increased Treg proportions, reduced DC and effector T cell activation, and lower responses to IL-2 (85), although they were also found to be associated with longer progression-free survival during anti-PD-1 treatment (86). Moreover, prebiotic mucin increasing ex vivo outgrowth of A. muciniphila decreased growth kinetics of aggressive melanoma in a gut microbiota and T cell-dependent manner, reducing serum levels of pro-inflammatory and immunosuppressive IL-6, IL-1α, IL-10, IL-17A, IL-23 cytokines (87); notably, prebiotic inulin operated through a different mode of action, facilitating the dominance of Bifidobacteria species in the intestines, boosting splenic cytotoxic T lymphocyte functions, and overcoming melanoma resistance to MEK inhibitors (87).
The effects of gut microbiota on the tumor microenvironment
The intestinal ecosystem can influence both local and distant neoplasia by impacting their immune context, influx of myeloid and lymphoid cells, and inflammatory and metabolic patterns. Secretory components of gut microbiota can be important: for example, outer membrane vesicles (OMVs) can reprogram the tumor microenvironment (TME) towards a pro-TH1 pattern (CXCL10, IFNγ) (88), or metabolites including butyrate and niacin can mediate Gpr109a-dependent induction of IL-18 in colonic epithelium and suppress colitis and colon cancer (89).
Tumor-associated, NOX2-mediated, myeloid cell ROS production is reduced by antibiotic administration or germ-free status, reducing oxaliplatin’s capacity to mediate early tumor genotoxicity (90). Similarly, commensal microbiota primed tumor-associated innate myeloid cells for TNFα (IL-1β, IL-12 and Cxcl10) production in response to anti-IL-10R/CpG-ODN treatment, and antibiotics, germ-free, or TLR4−/− status attenuated this response and the TNF-dependent early tumor necrosis (90). Supporting the adjuvant role of commensals against developing cancers, pasteurized A. muciniphila or its pili-like TLR2 agonist blunted azoxymethane-induced colitis and colon carcinogenesis by inducing TNFα-producing cytotoxic T lymphocytes in mesenteric lymph nodes (mLNs) that eventually reached the colonic mucosa (91). Spontaneous gut bacterial translocation in Tet2−/− mice also drove pre-leukemic myeloproliferation (PMP), which leads to leukemia if unchecked, in an IL-6-dependent manner (47). PMP was reversible with antibiotics and abolished in germ-free mice, suggesting new clinical management opportunities. However, an intact gut microbiome was later shown to be necessary to prevent leukemia progression in genetically predisposed mice (92).
Non-hematopoietic components of the intestinal mucosa are also linked to the TME (77). Gene deficient mice and BM chimeras identified a role for RNF5, an E3 ubiquitin ligase, in immunosurveillance of severe melanoma. Rnf5−/− mice exhibited decreased secretion of antimicrobial peptides and increased cell death in the ileal crypts, causing changes in intestinal microbiota community composition. This bowel injury amplified mobilization of CCR7-expressing DCs to Peyer’s patches, mLNs and melanoma-draining LNs, increasing IFNγ-producing T lymphocyte tumor infiltration. Confirming a Rnf5−/−-specific microbial effect, co-housing Rnf5−/− mice with wild type mice, or administering antibiotics, restored tumor aggressiveness while oral gavage with 11 species overrepresented in Rnf5−/− animals (Bacteroides and Parabacteroides spp.) into germ-free wild type mice recapitulated tumor immunosurveillance (77). In another study, oxaliplatin-induced caspase 3/7-dependent ileal apoptosis of crypts coincided with immunogenic bacteria dominance in the ileal mucosa (75). These commensals regulated TFH cell priming in mLNs, culminating in B cell activation, Ig production and infiltration of colon cancers with tumor-infiltrating lymphocytes (TILs) in mice and patients. Anti-CTLA-4-induced gut barrier dysfunction was also critical for systemic translocation of Bifidobacterium-derived inosine, in turn promoting TH1 activation and antitumor immunity by agonizing T cell-specific A2AR signaling in the context of DC costimulation (93). These examples illustrate that barrier injury is accompanied by a deviation of the local microbiome or translocation of microbial metabolites, that, in turn, mobilizes DCs to and outside the GALT and contributes to tumor bed infiltration by activated helper or cytotoxic T cells.
The TME comprises not only stromal, tumor, endothelial cells, and hematopoietic progenitor-derived immune components, but also a dense network of adrenergic nerve fibres that influence oncogenesis in brain and non-brain cancers (94–97). Interestingly, enteric nervous system neurons are both affected by the gut microbiota and functionally tuned according to their location in the gut. A subset of microbiota-responsive neurons could influence metabolic control independent of the central nervous system (98). These findings suggest intimate relationships between mucosal or tumoral commensals and tumor innervation that need further study.
Gut microbiota-mediated effects on anti-cancer drugs
Gut microbes are intimately involved in biotransformation of xenobiotics, including cancer drugs, with unintended consequences for clinical cancer control (99). For example, in prostate cancer, abiraterone acetate (AA) was used as an energy source by A. muciniphila and inhibited Corynebacterium species relying on AA-inhibited androgens for growth (100). Because A. muciniphila is anti-inflammatory and Corynebacterium species are pro-inflammatory, this change in their relative abundances increased pharmacologic efficacy of AA therapy. A. muciniphila’s immunomodulatory effects (78), including association with responders during PD-1 blockade (17), has prompted speculation that increased A. muciniphila may explain the efficacy of AA in androgen-independent prostate cancer (100), although this remains to be tested in large patient cohorts. These types of bidirectional drug-microbiota feedback loops warrant further study.
Intratumor microbiota effects on the tumor microenvironment
Mechanistic studies of live microbiota within diverse tumor types have been limited, particularly outside the aerodigestive tract, but many of their effects on the TME appear to suppress local antitumor immunity (15, 23, 34, 46, 75, 101, 102). Additionally, intratumor microbes have been reported to have cancer-specific effects on (i) gastrointestinal and urinary tract mutagenesis via secreted genotoxins, most notably pks+ E. coli-derived colibactin and B. fragilis-derived toxin (22, 37, 38, 40, 41); (ii) CagA-mediated or IL-17-producing γδ T cell-mediated inflammation in stomach and lung cancers, respectively (18, 103); (iii) chemoresistance via direct microbial metabolism (cytidine deaminase degradation of gemcitabine) in pancreatic cancer or indirect amplification of cancer cell autophagy in colorectal cancer (14, 104); (iv) tumor proliferation via fungal activation of the host’s C3 complement cascade in pancreatic cancer (20); and (v) metastasis through upregulating tumor matrix metalloproteinases in breast cancer or reducing tumor immunosurveillance in lung cancer (34, 46). Immunologically, intratumor microbes often create tolerogenic programming through PRR ligation with lower proportions of TILs, including CD8+ T cells, and occasionally more CD4+CD25+FoxP3+ Tregs, as observed in colorectal, pancreatic, breast, and lung cancers (18, 23, 34, 46, 75, 101, 102). However, in certain cases, injection of intratumoral bacteria or their antigens may conversely provide immunostimulatory effects, as demonstrated by Coley’s toxins and recent developments in bacterial cancer therapy (5, 105, 106). In breast cancer, experiments comparing SCID-beige and C57BL/6 mice with intratumor Fusobacterium suggested lymphoid-lineage cells as key mediators of intratumor microbiota-derived effects on tumor immunosurveillance (34). There are also associations between intratumor microbiota and immunogenicity, including differential melanoma immunotherapy response and triple-negative breast cancer associations, but their underlying mechanisms remain uncharacterized (12).
Extra-intestinal barriers and cancer microbiota
Given that the intestinal barrier offers the largest host-microbial interface and greatest microbial diversity, investigations on the potential impact of the microbiota in oncogenesis or cancer prognosis have primarily focused on this barrier (107). These studies could unveil cause-effect relationships between gut microbial composition changes and compromised tumor immunosurveillance, even in extraintestinal malignancies. However, it is noteworthy that extra-intestinal cancers can develop within tissues that harbor their own microbiome and may play a role in the exacerbation of neoplasia (12, 18, 25, 46).
For instance, the lungs’ surface approximates 1 m2/kg of body weight and is not sterile (108). Experimental evidence in oncogene-driven autochthonous lung cancer models in mice unveiled that local commensals may be perturbed by carcinogenesis, triggering an inflammatory cross-talk between alveolar macrophages and IL-17 producing lung resident γδ T cells contributing to tumor progression (18). The clinical significance of this observation has been recently brought up in 83 lung cancer patients (25). Tsay et al. highlighted that microaspiration of supraglottic commensals in lung cancer patients can affect response to therapies and overall survival, due to a TH17-mediated exacerbated inflammation corollary to immune checkpoint inhibition (25).
Skin is also recognized as our largest and outermost organ, maintaining host homeostasis through tight interconnections between its resident microbes, keratinocytes, and skin immune components through metabolic, innate, and cognate immune responses (109). Compositional shifts in the skin microbiota appear to influence non-melanoma skin carcinogenesis (110). Similarly, cervical cancer caused by persistent high-risk human papillomavirus infection is often associated with a deviated cervical microflora (111, 112). The intertwined/interkingdom relationships between commensals and virus-associated cancers, and their synergistic effects on tumorigenesis need further study, and exploration of cancer-microbe interactions at other extra-intestinal barriers is warranted.
Cancer microbiome diagnostics
Variation in human microbiome composition among body sites (113) contrasts with stable human genetics exhibiting only minor variation resulting from somatic mosaicism and clonal hematopoiesis (114). Because both host tissues and microbiota are affected by carcinogenesis, the genetic heterogeneity of microbes may provide an opportunity to diagnose and locate disease. For example, a blood-derived TP53 mutation can indicate host cancer status but implicates >25 cancer types (115); conversely, Streptococcus gallolyticus (formerly S. bovis) bacteremia can reflect host cancer status and type (colon cancer) based on its gastrointestinal origin (116, 117). Many challenges exist for microbial-based diagnostics, including low biomass relative to host and confounding from reagent or environmental contaminants. Many questions about their uniqueness, prevalence, stability during cancer treatment, or utility during antibiotic administration remain to be answered and must be addressed before clinical deployment.
Nearly all microbial-based cancer diagnostics are sequencing-based and have focused on tumors within the aerodigestive tract (31), such as colorectal (118–121), pancreatic (122, 123), and lung cancer (124–126). It was only recently suggested that cancer types outside of the aerodigestive tract, such as breast or brain cancer, may also harbor microbiota with unique compositions. Nejman et al. (12) and Poore et al. (13) suggest distinct intratumoral microbiomes among >30 cancer types (Fig. 3), proposing their applicability to blood-based diagnostics and providing imaging evidence of these microbes’ intratumoral spatial distribution and intracellular localization in seven cancer types, although imaging evidence remains lacking for most cancer types.
Fig. 3. Current landscape of the cancer microbiome.
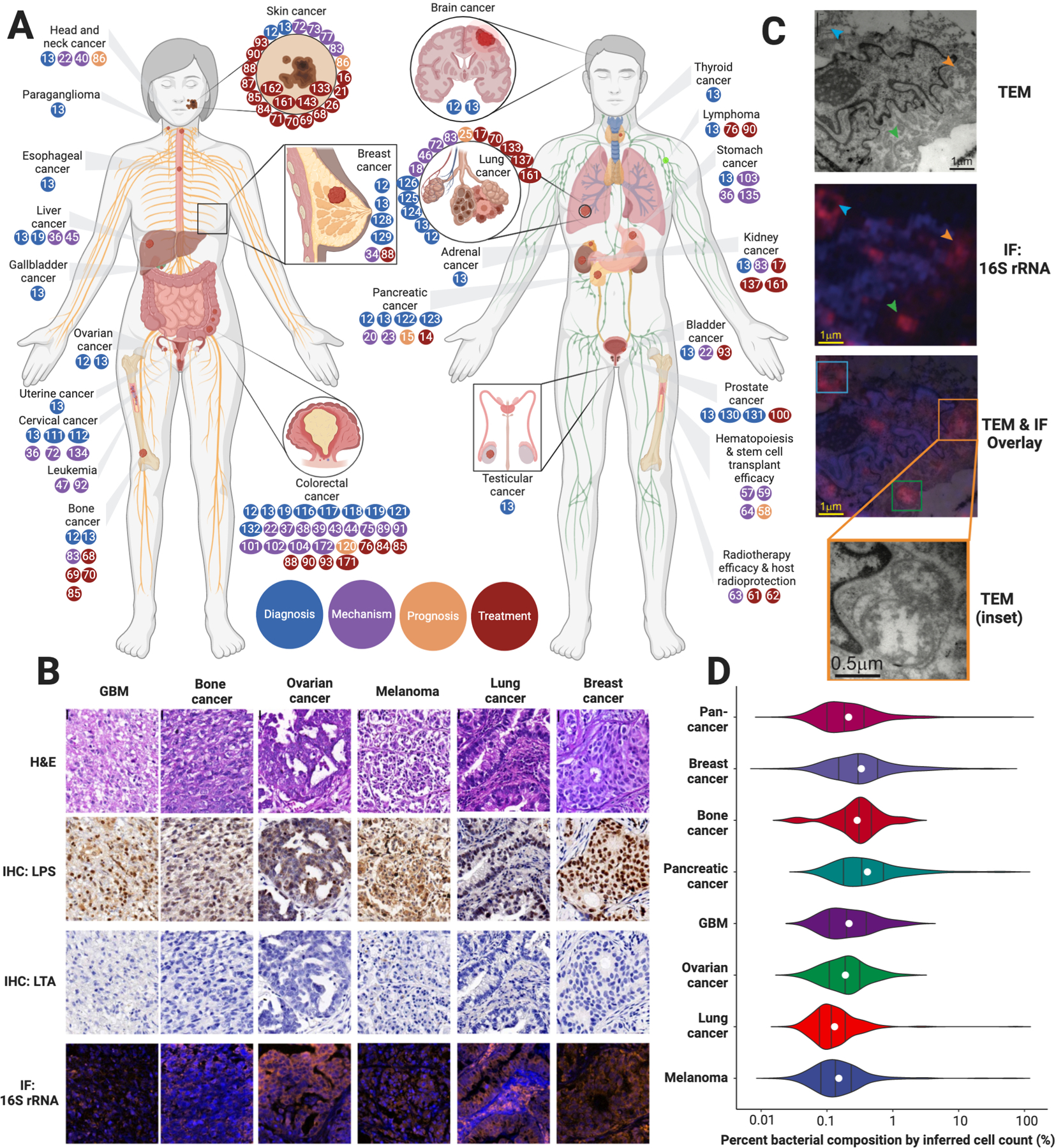
(A) Body diagram of all cancers currently linked to microbiota, where the colored dots reflect reference numbers and are colored according to the major theme of the referenced paper (diagnosis, mechanism, prognosis, treatment). Dots are included based on existing preclinical and clinical data. (B) Representative histology, immunohistochemistry (IHC) for lipopolysaccharide (LPS) and lipoteichoic acid (LTA), and immunofluorescence (IF) for bacterial 16S rRNA in six cancers. (C) Representative transmission electron microscopy (TEM) images with overlaid 16S rRNA immunofluorescence of intracellular bacteria (arrows) in breast cancer. (D) Estimation of tumor percent bacterial composition across seven cancer types assuming tissue homogeneity and 8 picograms of DNA per cancer cell. Black lines depict distributional quantiles (25%–50%–75%); white dots reflect averages. (B-D) Adapted from Nejman et al. (12)
Combining multi-region 16S rRNA amplicon sequencing, qPCR, immunohistochemistry (lipopolysaccharide [LPS], lipoteichoic acid [LTA]), immunofluorescence (16S rRNA), cultivation, and electron microscopy, Nejman et al. (12) surveyed 1010 tumors for bacteria across melanoma, lung, ovarian, glioblastoma, pancreas, bone, and breast cancers. They included 811 experimental controls, covering DNA extraction, PCR amplification, and paraffin embedding, removing 94.3% of bacteria as contaminants. Examining the residual 528 bacterial species revealed significant differences in composition, diversity, and inferred metabolic functionality between cancer types. Histologic imaging revealed heterogeneous microbial spatial distributions (Fig. 3B) and their frequent intracellular localization in cancer and immune cells (Fig. 3C). As described above in the Overview of the cancer microbiome section, qPCR estimated the number of bacteria per tissue section, which we have graphically depicted as percent bacterial composition per cancer type assuming tissue homogeneity and 8 picograms of DNA per cancer cell (Fig. 3D). Applying their pipeline to a melanoma immunotherapy cohort suggested microbiome differences between responders and non-responders, but not yet a mechanism. Because bacteria were cultured from only five human breast tumors, the widespread viability of intratumoral bacteria from this study was unclear, particularly in cancers with reportedly fewer bacteria. However, other studies have indeed shown cultivable bacteria in breast (127–129), lung (18), prostate (130, 131), pancreas (14, 15), and colon cancers (19, 132), suggesting broad microbial viability. Still, basic questions remain about the functional impacts of these intratumoral microbiota and whether they are parasitic, symbiotic, or passive passengers, and a biopsy specimen is required for analysis, limiting its diagnostic utility.
Poore et al. (13) took a different approach by harvesting all treatment-naive whole genome and transcriptome studies from The Cancer Genome Atlas (TCGA) (n=18,116 samples; 33 cancer types) to study bacterial, viral, and archaeal nucleic acids. Because no experimental controls were available, they filtered out historically-known reagent contaminants and inferred other contaminants using per-sample DNA and RNA concentrations; these steps removed up to 91.3% of microbial taxa. Machine learning revealed intratumor, cancer-specific microbial signatures. Because colon cancer is epidemiologically linked to clinical bacteremia (116, 117), they explored TCGA blood-derived normal samples (n=1,866 samples) for cancer-specific microbial DNA and reported highly-accurate cancer discrimination. They validated this blood-based diagnostic approach by comparing plasma-derived cell-free microbial DNA from 100 patients with lung, prostate, or melanoma cancers to those from 69 HIV-negative, healthy patients while implementing necessary experimental contamination controls. Although closer to a practical diagnostic approach, the absence of experimental controls in TCGA, sole reliance on deep sequencing data without orthogonal approaches, and current lack of explainable mechanism(s) by which microbial DNA enters into and survives circulation limits these findings. We speculate that the intracellular bacteria in cancer and immune cells identified by Nejman et al. (12) may provide one source, though this remains to be demonstrated. A rigorous evaluation alongside blood samples from patients with non-lethal bodily infections, septic patients, and patients receiving antibiotics during cancer care are necessary preconditions to broad clinical utility.
Modulation of the cancer microbiome
The associations between certain gastrointestinal microbiota and the activity of systemic lymphoid tissues have stimulated interest in microbial modulation as a powerful immunotherapeutic modality. If intratumoral microbiota are eventually verified to be prevalent and immunologically active across most patients, as preliminary data suggest (12, 13), such interventions must account for microbial niches and their crosstalk (Fig. 4). These dynamics sometimes appear related; for example, modulation of gut microbiota influences the composition of the intratumoral microbiome in pancreatic cancer, presumably via pancreatic duct communication (15, 20, 23). However, in other cases, these changes are incongruent; for instance, antibiotics appear to abrogate immunotherapy response by inhibiting the gut microbiome (133), but paradoxically they improve immunotherapy efficacy by upregulating PD-1 expression when eliminating the pancreatic intratumoral microbiome (23). These complexities necessitate more in-depth mechanistic studies of modulation approaches and better clinical understanding before applying prebiotics, probiotics, postbiotics, and antibiotics in the setting of cancer.
Fig. 4. Considerations when modulating the endogenous cancer microbiome.
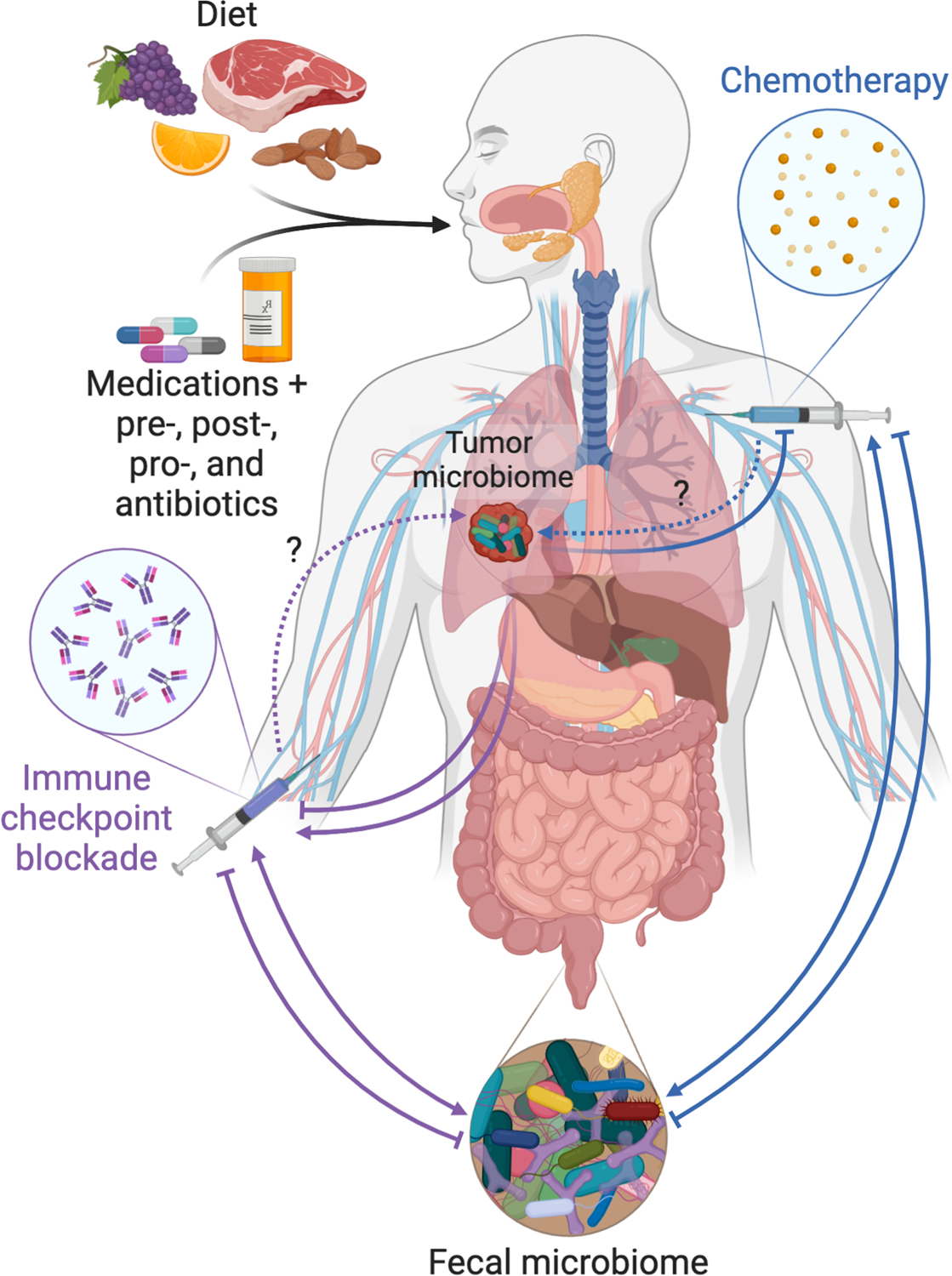
Diet, medications, and prebiotics, postbiotics, probiotics, and antibiotics all have the capacity to modify the gut and tumor microbiomes. Bi-directional influences may exist between these microbiomes and cancer therapies (chemotherapy and immunotherapy). For instance, chemotherapy can cause compositional changes in the gut microbiome, which in turn enhance treatment efficacy (75); in other cases, chemotherapy may be degraded by microbes (14). Thus, modification of the gut and/or tumor microbiomes may be advantageous for one modality of therapy while disadvantageous for another. Dotted arrows denote gaps in the literature.
Antibiotics and the cancer microbiome
The use of antimicrobial therapy in cancer is limited to addressing or preventing known microbial carcinogens. This includes treating H. pylori-derived gastric lymphomas with triple or quadruple antibiotic therapy, administering direct-acting antivirals against active Hepatitis C virus, and vaccinating against major human papillomavirus serotypes and hepatitis B virus to prevent urogenital, cervical, head and neck, and liver cancers (36, 134, 135). Excluding antibiotic-derived chemotherapies (e.g. doxorubicin), there is circumstantial and conflicting evidence for the use of antibiotics in solid tumors. Several studies in lung, colon, and pancreatic cancer suggest that eliminating intratumoral microbiota can check tumor-promoting inflammatory processes, reduce cellular proliferation, or convert a tolerogenic TME to an immunogenic one (18, 19, 23, 46). However, increasing clinical evidence suggests that systemic antibiotics abolish immune checkpoint blockade efficacy and decrease patient survival (133, 136, 137). In hematologic malignancies, preclinical evidence suggests a careful balance, where either antibiotics or gut bacterial translocation can trigger leukemic progression in genetically-predisposed hosts (47, 92).
Prebiotics, postbiotics, and dietary interventions to modify the microbiome are also promising. Dietary effects on cancer were recently reviewed in detail, with many epidemiological associations but few causal mechanisms (138). Difficulties in dietary data collection have impeded strong conclusions, but metabolomic data that can reveal dietary intake and concomitant small molecule effectors may help in the future. Prebiotics (molecules that promote growth of beneficial microbes) such as resistant starch, inulin, and mucin are promising in preclinical models, improving antitumor immunity and therapy response in melanoma and colon cancer (87), and are in clinical trials (e.g. NCT03870607, NCT03950635). Experimental evidence of postbiotic compounds (microbial-derived molecules) is limited in cancer, but they may provide advantages through defined composition and manufacturing reproducibility (139).
Gut microbiota can also be modulated in cancer through fecal microbiota transplantation (FMT), administration of defined microbial consortia, and commercial probiotics. FMT treats Clostridium difficile (now Clostridioides difficile) colitis effectively (140), with some efficacy in the treatment of immunotherapy-associated colitis (141). The long-term efficacy and stability of FMT remain unknown (142). Targeting gut microbes clinically is complicated by factors such as antibiotic pre-conditioning, administration route, frequency of modulation, and dietary recommendations (142). Ongoing clinical trials suggest that FMT from donors responsive to immunotherapy may enhance antitumor immune and potentially clinical responses (NCT03353402, (143)). Additional clinical trials are evaluating the impact of transferring microbial consortia, ranging in complexity from monoclonal bacterial strains to multiplexed consortia. Few commercially available probiotic formulations have been tested for impact on antitumor and systemic immunity, with certain formulations actually increasing tumorigenesis (144). In critically ill patients, commercial probiotic use may even cause bacteremia (145). Therefore, indiscriminate administration of commercially available probiotics in cancer patients should be discouraged.
Cancer therapy using exogenous microbiota
Major strides have been made towards engineering exogenous bacterial and viral agents for cancer therapy, particularly as powerful immunotherapy options or neoadjuvants. Two such agents have FDA approval: oncolytic viral therapy for advanced melanoma using talimogene laherparepvec (T-VEC) (146), and bacterial cancer therapy for high-risk, non-muscle invasive bladder cancer using live-attenuated Mycobacterium bovis (BCG vaccine) (147). Because oncolytic viruses are non-commensals and have been reviewed elsewhere in detail (148–150), we focus our attention on bacterial cancer therapies (BCTs). Though historically contentious, BCT is re-gaining attention through synthetic biology techniques that programmatically limit systemic toxicities while enhancing regional antitumor immunity (105, 106). Regulatory challenges for BCT agents are considerable (Fig. 5A), and despite ongoing clinical trials (e.g. NCT04167137), they have yet to be commercially surmounted (151).
Fig. 5. Synthetic biology for exogenous cancer therapeutics.
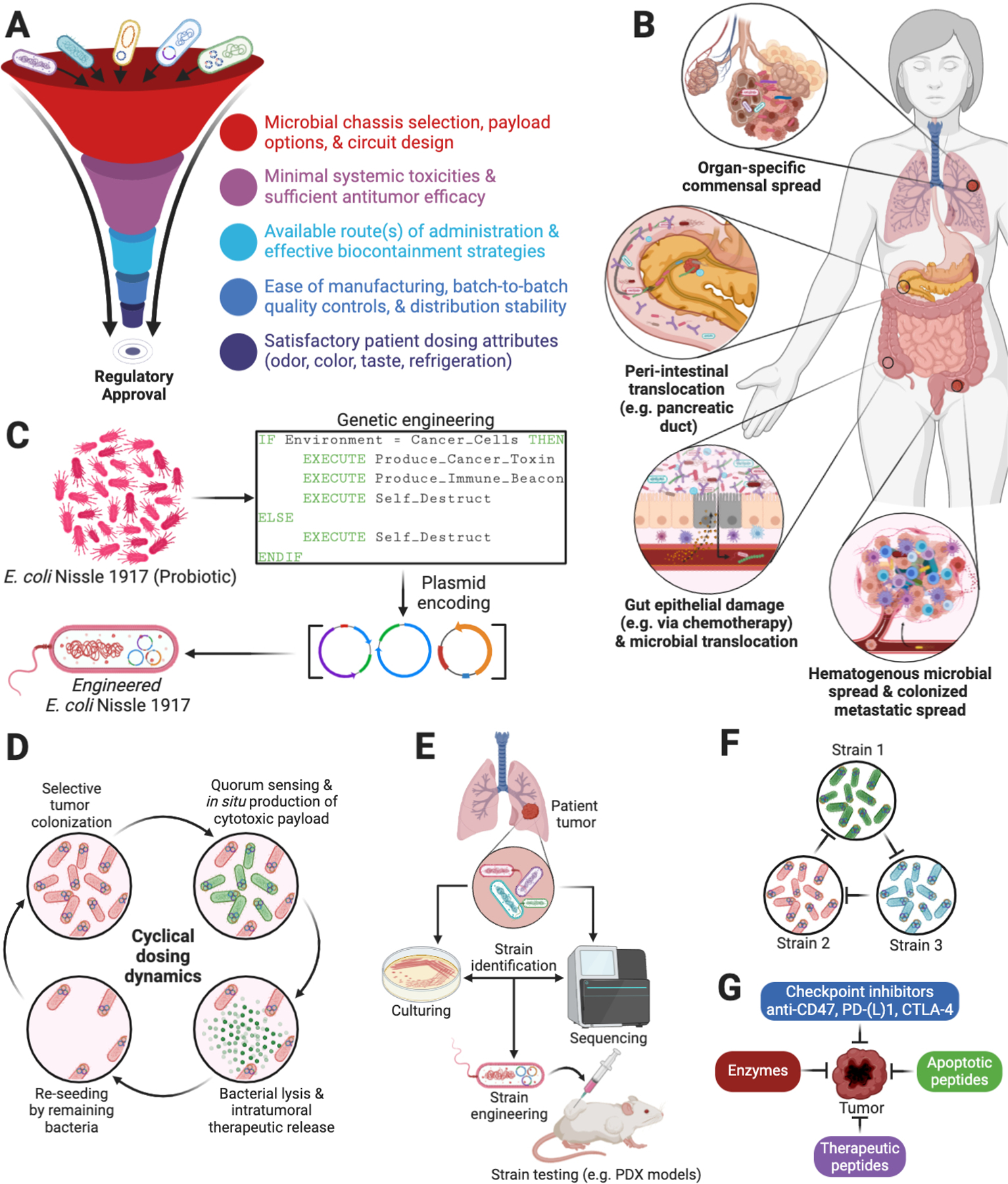
(A) Regulatory considerations for engineering bacteria against cancer (151). (B) Diverse sources of intratumoral bacteria include organ-specific commensals (18, 19, 25, 46, 112), gut communication (15, 20, 23), hematogenous spread (171, 172), and intra-metastatic spread (19). (C) Some probiotics, such as E. coli Nissle 1917, possess strong safety records (173), have been shown to naturally migrate to solid tumors in animal models, and can be programmed to produce and deliver therapies from within solid tumors (155). (D) Complex population dynamics can be engineered to generate the cyclical delivery of therapeutics (155, 156, 174). (E) Future efforts will likely center on engineering and testing strains that are found naturally in patient-specific tumors. (F) Engineered ecologies can be designed to create tailored, tumor-specific therapeutic cocktails (175, 176). (G) Multiple drug payloads can be encoded by one or more engineered strains against tumors.
Engineered microbes as cancer drugs
Natural bacterial mechanisms for tumor tropism are numerous (Fig. 5B), with intratumoral or intravenous injection often leading to ~10,000-fold accumulation in tumors relative to matched liver, spleen, and lung tissues (152, 153). This affinity for tumor tissue provides a creative drug chassis and natural bridge from synthetic biology to cancer therapies, whereby cytotoxic payloads can be encoded for programmed delivery by tumor-homing bacteria (Figs. 5C–G). Thus far, genetically attenuated, auxotrophic, and inducible versions of Escherichia, Bifidobacterium, Listeria, Shigella, Clostridium, Lactococcus, Vibrio, and Salmonella species have been engineered and shown antitumor efficacy in preclinical models with intravenous, intratumor, and oral delivery routes (147). While some approaches are based on intracellular delivery of drugs via phagocytic uptake of bacteria, others program bacteria to act as “intratumoral bioreactors” that continuously produce and release payloads extracellularly as part of colonization. An interesting general approach implements engineered bacterial lysis, which enables antitumor protein production or release only when a predefined population density of bacteria is reached (154–157). This dramatically reduces bacterial colony size and prevents systemic toxicities. Din and colleagues were the first to demonstrate how non-pathogenic E. coli and Salmonella could be engineered to lyse at a threshold population density, releasing a chemokine, hemolysin, or pro-apoptotic protein, or all three, into the TME at desired periodic intervals (155). The drugs are delivered cyclically as the bacterial population is programmed to generate growth-death-regrowth cycles. Chowdhury et al. then used this design to produce and release an antibody-fragment nanobody against CD47, which tumors can overexpress to inhibit DC phagocytosis (156). Intriguingly, this stimulated a tumor-antigen-specific CD8+ T cell response that prevented metastasis and mediated an abscopal effect, which regressed distal non-injected tumors as well. This approach further precluded host anemias and thrombocytopenias usually seen with systemic CD47 antagonism, suggesting a clinical opportunity. If intratumoral bacteria prove to be prevalent across various cancer types, lysis circuit designs may also provide an opportunity to flexibly engineer patient-specific, tumor-specific commensal strains (Fig. 5E) or several strains in feedback with each other (Fig. 5F) to regulate payload release. Given the many encodable cytotoxic payloads (Figs. 5G), a clear demonstration of BCT clinical efficacy with minimal systemic toxicities could considerably increase the cancer therapy armamentarium.
Outlook for the cancer microbiome
The last 15 years of microbiome research provide intriguing, though still controversial, evidence of the relationships between microbes and cancer and the nuances of these relationships. Few microbes directly cause cancer, but many more seem complicit, and, perhaps counterintuitively, several promote host antitumor immunity. This complexity may reflect shared evolutionary dynamics between the host’s immune system, its commensal microbiota, and tumorigenic processes that we are just beginning to uncover (158–160).
A substantial literature gap still separates clinical observations and clinical interventions targeted at microbiota in cancer. Although gut microbiota modulation in murine immunotherapy models provides tantalizing results, they have not yet translated to commercial therapeutic interventions in humans. Moreover, observations in humans and mice of gut microbes that stratify therapy response, particularly immunotherapy (16, 17, 21, 26), have not uncommonly shown varying taxonomic differences that persist despite uniform bioinformatic re-analyses, although there is greater concordance when examining functional profiles (161, 162). Thus, many of the key problems that plagued researchers in the early 20th century — contamination, irreproducibility, patient toxicities — remain challenges today for microbially-based cancer diagnostics, prognostics, and exogenous microbial therapeutics. Additional cohorts with carefully curated samples to limit and mitigate potential contamination are needed to help characterize and understand the impact of intratumoral microbes on carcinogenesis, cancer progression, and therapy response. Other efforts are needed to examine non-bacterial relationships with cancer, gastrointestinally and intratumorally, and their functions, particularly in relationship with known bacterial functions. Further consortium-level efforts are necessary to assess the quantitative impact of technical variables (e.g. DNA extraction, sample handling, bioinformatic protocols) on cancer microbiome data and guide the selection of “gold-standard” pipelines, analogous to the Microbiome Quality Control consortium’s analysis of fecal amplicon sequencing among 15 laboratories and nine bioinformatic protocols (163).
Many of these challenges would be aided by a multi-center, longitudinal, concerted effort to study microbiota in cancer, analogous to TCGA’s role in elucidating the somatic mutation landscape, with joint tumor, blood, and stool collection, multi-omic data generation, and incorporation of experimental contamination controls (Fig. 6) (164). Concurrent meta-analyses of existent cancer datasets with uniform in silico host depletion, decontamination, taxonomy calling, and functional profiling may be able to identify global microbial drivers in cancer pathogenesis and treatment despite technical variation between individual studies (13, 118, 119, 165–167). Completion of microbiota modulation trials are additionally crucial for guiding clinical applications and increasing the cancer therapy armamentarium (142), with new evidence demonstrating that modulation of the gut microbiota using FMT in immunotherapy-refractory melanoma patients is associated with clinical responses and changes in the gut and tumor immune microenvironment (143) (Davar et al., In press). In-depth functional analyses at community and per-microbe scales are likely necessary to elucidate microbial-immune-cancer cell mechanistic interactions and emerging spatial multi-omic tools may prove invaluable here (168, 169). Engineered organoids with immune and microbiota niches or metabolites may further help validate or refute microbial causality or complicity in carcinogenesis, as recently demonstrated by colibactin mutagenesis studies (22, 170). In sum, although many challenges remain, building a better understanding of the roles of microbes in cancer may enable a powerful new toolkit for improving patient care.
Fig. 6. Study design for characterizing cancer-associated microbiota and their functional impacts.
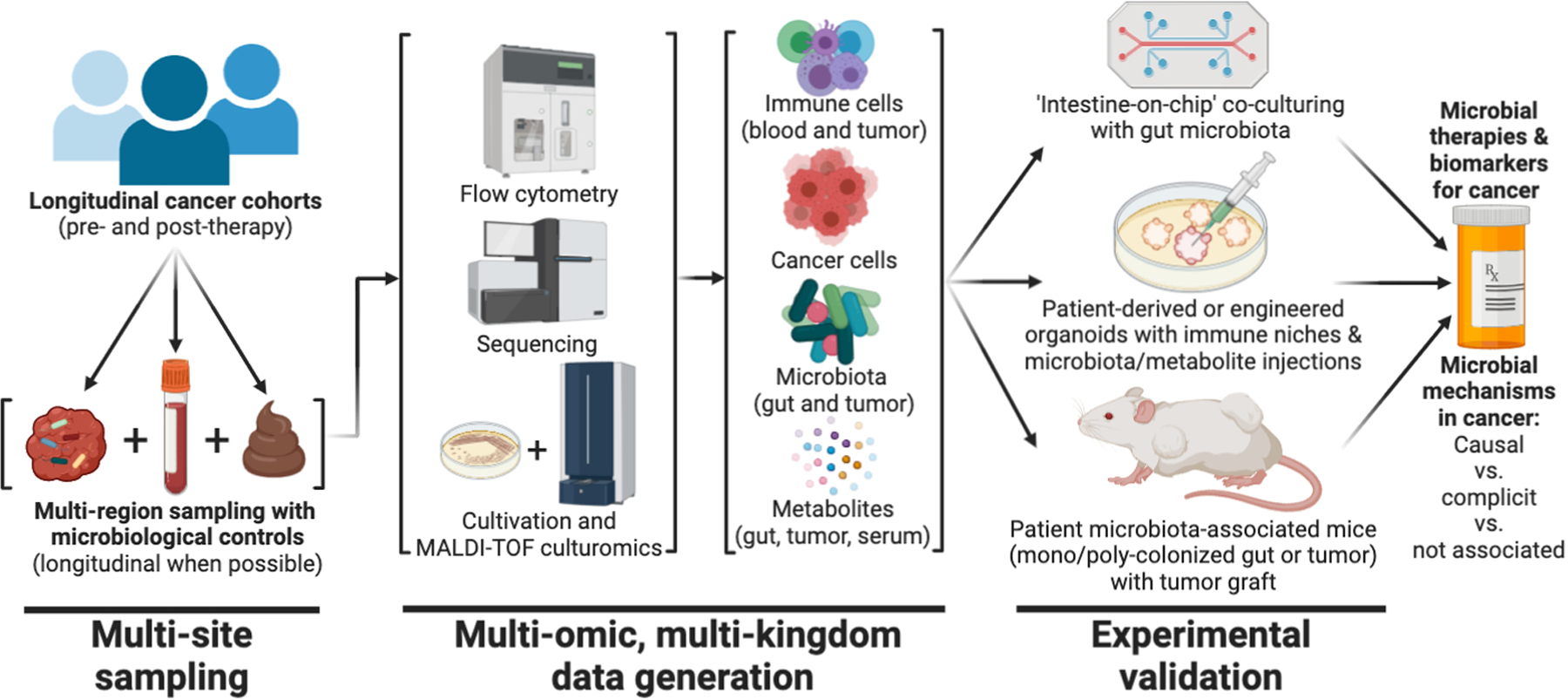
Opportunities exist to perform large-scale identification of the presence and function of cancer-associated microbiota, beginning with longitudinal cohorts and multi-region sampling. Existing tools can be used to gather multi-omic information on host immune cells, cancer cells, microbiota, and metabolites (51, 177, 178). In vitro and in vivo disease models of a patient’s tumor and intestine can then be used to verify or rebut the predicted functional impact and mechanism(s) of a given microbe (or its metabolites) and its causality in carcinogenesis (160, 170).
ACKNOWLEDGEMENTS
We thank C. Sepich-Poore (University of Chicago), R. Sullivan (Massachusetts General Hospital), and J. Mesirov (University of California San Diego) for providing critical review and feedback on the manuscript and figures. Figures were created with BioRender.com. The authors also wish to acknowledge the patients and their families who have helped contribute towards a better understanding of this field.
Funding:
G.D.S-P. is supported by a fellowship from the US National Institutes of Health, National Cancer Institute (F30 CA243480). J.H. and R.K. are supported by the National Cancer Institute of the National Institutes of Health under award number R01CA255206. The L.Z. laboratory is supported by RHU Torino Lumière (ANR-16-RHUS-0008), the ONCOBIOME project (European Union Horizon 2020 programme), the Seerave Foundation, the French Agence Nationale de la Recherche (Ileobiome), the French Ligue Contre le Cancer (Équipe Labelisées programme), the French Association pour la Recherche sur le Cancer, Cancéropôle Ile-de-France, the French Fondation pour la Recherche Médicale, a donation by Elior and Dassault Systems, the European Research Council, Fondation Carrefour, the French Institut National du Cancer INSERM (HTE programme), LabEx Immuno-Oncology, SIRIC Stratified Oncology Cell DNA Repair and Tumour Immune Elimination (SOCRATE) and the CARE network (directed by X. Mariette, Assistance Publique — Hôpitaux de Paris, Kremlin-Bicêtre).
Competing interests:
G.D.S-P. and R.K. are inventors on a US patent application (PCT/US2019/059647) submitted by The Regents of the University of California and licensed by Micronoma that covers methods of diagnosing and treating cancer using microbial biomarkers in blood and cancer tissues. G.D.S-P. and R.K. are founders of and report stock interest in Micronoma. R.K. additionally is a member of the scientific advisory board for GenCirq, holds an equity interest in GenCirq, and can receive reimbursements for expenses up to US$5,000 per year. R.S. received a grant from Merck EMD Serono, is a member of the scientific advisory board for Micronoma, and is a paid adviser to Biomica and BiomX. R.S. is also a co-inventor on a U.S. provisional patent application (63/005,540) submitted by Yeda Research and Development, the Weizmann Institute of Science, that covers methods of diagnosing and treating cancer using microbial biomarkers in cancer tissues. J.H. is a founder of and has a financial interest in GenCirq, which focuses on cancer therapeutics. J.W. is an inventor on a US patent application (PCT/US17/53.717) submitted by the University of Texas MD Anderson Cancer Center that covers methods to enhance immune checkpoint blockade responses by modulating the microbiome. J.W. further reports compensation and honoraria from Imedex, Dava Oncology, Omniprex, Ilumina, Gilead, PeerView, Physician Education Resource, MedImmune and Bristol-Myers Squibb. J.W. serves as a consultant advisory board member for Roche/Genentech, Novartis, AstraZeneca, GlaxoSmithKline, Bristol-Myers Squibb, Merck, Biothera Pharmaceuticals and Microbiome DX. J.W. also receives research support from GlaxoSmithKline, Roche/Genentech, Bristol-Myers Squibb and Novartis. J.W. is an adviser and has stock options for Ella Therapeutics. L.Z. is a founder of everImmune, a biotechnology company that develops anticancer probiotics and diagnostic tools to define intestinal dysbiosis in cancer. L.Z. also has an active scientific collaboration (research contract) with Kaleido, Innovate Pharma, and Bioaster, which are companies involved in the microbiome space.
REFERENCES AND NOTES
- 1.Ebbell B, The Papyrus Ebers: the greatest Egyptian medical document (Levin & Munksgaard, 1937). [Google Scholar]
- 2.Hoption Cann SA, van Netten JP, van Netten C, Dr William Coley and tumour regression: a place in history or in the future. Postgrad. Med. J 79, 672–680 (2003). [PMC free article] [PubMed] [Google Scholar]
- 3.Busch W, Aus der Sitzung der medicinischen Section vom 13 November 1867. Berl Klin Wochenschr. 5, 137 (1868). [Google Scholar]
- 4.Fehleisen F, Ueber die Züchtung der Erysipelkokken auf künstlichem Nährboden und ihre Übertragbarkeit auf den Menschen. Dtsch. Med. Wochenschr 8, 553–554 (1882). [Google Scholar]
- 5.Starnes CO, Coley’s toxins in perspective. Nature. 357, 11–12 (1992). [DOI] [PubMed] [Google Scholar]
- 6.Glover TJ, The bacteriology of cancer. Canada Lancet Pract. 75, 92–111 (1930). [Google Scholar]
- 7.Livingston-Wheeler therapy. CA Cancer J. Clin 40, 103–108 (1990). [DOI] [PubMed] [Google Scholar]
- 8.Rous P, A SARCOMA OF THE FOWL TRANSMISSIBLE BY AN AGENT SEPARABLE FROM THE TUMOR CELLS. J. Exp. Med 13, 397–411 (1911). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White MK, Pagano JS, Khalili K, Viruses and human cancers: a long road of discovery of molecular paradigms. Clin. Microbiol. Rev 27, 463–481 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanahan D, Weinberg RA, The hallmarks of cancer. Cell. 100, 57–70 (2000). [DOI] [PubMed] [Google Scholar]
- 11.Hanahan D, Weinberg RA, Hallmarks of cancer: the next generation. Cell. 144, 646–674 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Nejman D, Livyatan I, Fuks G, Gavert N, Zwang Y, Geller LT, Rotter-Maskowitz A, Weiser R, Mallel G, Gigi E, Meltser A, Douglas GM, Kamer I, Gopalakrishnan V, Dadosh T, Levin-Zaidman S, Avnet S, Atlan T, Cooper ZA, Arora R, Cogdill AP, Khan MAW, Ologun G, Bussi Y, Weinberger A, Lotan-Pompan M, Golani O, Perry G, Rokah M, Bahar-Shany K, Rozeman EA, Blank CU, Ronai A, Shaoul R, Amit A, Dorfman T, Kremer R, Cohen ZR, Harnof S, Siegal T, Yehuda-Shnaidman E, Gal-Yam EN, Shapira H, Baldini N, Langille MGI, Ben-Nun A, Kaufman B, Nissan A, Golan T, Dadiani M, Levanon K, Bar J, Yust-Katz S, Barshack I, Peeper DS, Raz DJ, Segal E, Wargo JA, Sandbank J, Shental N, Straussman R, The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science. 368, 973–980 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poore GD, Kopylova E, Zhu Q, Carpenter C, Fraraccio S, Wandro S, Kosciolek T, Janssen S, Metcalf J, Song SJ, Kanbar J, Miller-Montgomery S, Heaton R, Mckay R, Patel SP, Swafford AD, Knight R, Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature. 579, 567–574 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geller LT, Barzily-Rokni M, Danino T, Jonas OH, Shental N, Nejman D, Gavert N, Zwang Y, Cooper ZA, Shee K, Thaiss CA, Reuben A, Livny J, Avraham R, Frederick DT, Ligorio M, Chatman K, Johnston SE, Mosher CM, Brandis A, Fuks G, Gurbatri C, Gopalakrishnan V, Kim M, Hurd MW, Katz M, Fleming J, Maitra A, Smith DA, Skalak M, Bu J, Michaud M, Trauger SA, Barshack I, Golan T, Sandbank J, Flaherty KT, Mandinova A, Garrett WS, Thayer SP, Ferrone CR, Huttenhower C, Bhatia SN, Gevers D, Wargo JA, Golub TR, Straussman R, Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science. 357, 1156–1160 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riquelme E, Zhang Y, Zhang L, Montiel M, Zoltan M, Dong W, Quesada P, Sahin I, Chandra V, San Lucas A, Scheet P, Xu H, Hanash SM, Feng L, Burks JK, Do K-A, Peterson CB, Nejman D, Tzeng C-WD, Kim MP, Sears CL, Ajami N, Petrosino J, Wood LD, Maitra A, Straussman R, Katz M, White JR, Jenq R, Wargo J, McAllister F, Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell. 178, 795–806.e12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, Cogdill AP, Zhao L, Hudgens CW, Hutchinson DS, Manzo T, Petaccia de Macedo M, Cotechini T, Kumar T, Chen WS, Reddy SM, Szczepaniak Sloane R, Galloway-Pena J, Jiang H, Chen PL, Shpall EJ, Rezvani K, Alousi AM, Chemaly RF, Shelburne S, Vence LM, Okhuysen PC, Jensen VB, Swennes AG, McAllister F, Marcelo Riquelme Sanchez E, Zhang Y, Le Chatelier E, Zitvogel L, Pons N, Austin-Breneman JL, Haydu LE, Burton EM, Gardner JM, Sirmans E, Hu J, Lazar AJ, Tsujikawa T, Diab A, Tawbi H, Glitza IC, Hwu WJ, Patel SP, Woodman SE, Amaria RN, Davies MA, Gershenwald JE, Hwu P, Lee JE, Zhang J, Coussens LM, Cooper ZA, Futreal PA, Daniel CR, Ajami NJ, Petrosino JF, Tetzlaff MT, Sharma P, Allison JP, Jenq RR, Wargo JA, Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 359, 97–103 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, Fidelle M, Flament C, Poirier-Colame V, Opolon P, Klein C, Iribarren K, Mondragón L, Jacquelot N, Qu B, Ferrere G, Clémenson C, Mezquita L, Masip JR, Naltet C, Brosseau S, Kaderbhai C, Richard C, Rizvi H, Levenez F, Galleron N, Quinquis B, Pons N, Ryffel B, Minard-Colin V, Gonin P, Soria J-C, Deutsch E, Loriot Y, Ghiringhelli F, Zalcman G, Goldwasser F, Escudier B, Hellmann MD, Eggermont A, Raoult D, Albiges L, Kroemer G, Zitvogel L, Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 359, 91–97 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Jin C, Lagoudas GK, Zhao C, Bullman S, Bhutkar A, Hu B, Ameh S, Sandel D, Liang XS, Mazzilli S, Whary MT, Meyerson M, Germain R, Blainey PC, Fox JG, Jacks T, Commensal Microbiota Promote Lung Cancer Development via γδ T Cells. Cell. 176, 998–1013.e16 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bullman S, Pedamallu CS, Sicinska E, Clancy TE, Zhang X, Cai D, Neuberg D, Huang K, Guevara F, Nelson T, Chipashvili O, Hagan T, Walker M, Ramachandran A, Diosdado B, Serna G, Mulet N, Landolfi S, Ramon Y Cajal S, Fasani R, Aguirre AJ, Ng K, Élez E, Ogino S, Tabernero J, Fuchs CS, Hahn WC, Nuciforo P, Meyerson M, Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science. 358, 1443–1448 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aykut B, Pushalkar S, Chen R, Li Q, Abengozar R, Kim JI, Shadaloey SA, Wu D, Preiss P, Verma N, Guo Y, Saxena A, Vardhan M, Diskin B, Wang W, Leinwand J, Kurz E, Kochen Rossi JA, Hundeyin M, Zambrinis C, Li X, Saxena D, Miller G, The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature. 574, 264–267 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre M-L, Luke JJ, Gajewski TF, The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 359, 104–108 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pleguezuelos-Manzano C, Puschhof J, Rosendahl Huber A, van Hoeck A, Wood HM, Nomburg J, Gurjao C, Manders F, Dalmasso G, Stege PB, Paganelli FL, Geurts MH, Beumer J, Mizutani T, Miao Y, van der Linden R, van der Elst S, Genomics England Research Consortium, Garcia KC, Top J, Willems RJL, Giannakis M, Bonnet R, Quirke P, Meyerson M, Cuppen E, van Boxtel R, Clevers H, Mutational signature in colorectal cancer caused by genotoxic pks E. coli. Nature. 580, 269–273 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pushalkar S, Hundeyin M, Daley D, Zambirinis CP, Kurz E, Mishra A, Mohan N, Aykut B, Usyk M, Torres LE, Werba G, Zhang K, Guo Y, Li Q, Akkad N, Lall S, Wadowski B, Gutierrez J, Kochen Rossi JA, Herzog JW, Diskin B, Torres-Hernandez A, Leinwand J, Wang W, Taunk PS, Savadkar S, Janal M, Saxena A, Li X, Cohen D, Sartor RB, Saxena D, Miller G, The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 8, 403–416 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fulbright LE, Ellermann M, Arthur JC, The microbiome and the hallmarks of cancer. PLoS Pathog. 13, e1006480 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsay J-CJ, Wu BG, Sulaiman I, Gershner K, Schluger R, Li Y, Yie T-A, Meyn P, Olsen E, Perez L, Franca B, Carpenito J, Iizumi T, El-Ashmawy M, Badri M, Morton JT, Shen N, He L, Michaud G, Rafeq S, Bessich JL, Smith RL, Sauthoff H, Felner K, Pillai R, Zavitsanou A-M, Koralov SB, Mezzano V, Loomis CA, Moreira AL, Moore W, Tsirigos A, Heguy A, Rom WN, Sterman DH, Pass HI, Clemente JC, Li H, Bonneau R, Wong K-K, Papagiannakopoulos T, Segal LN, Lower airway dysbiosis affects lung cancer progression. Cancer Discov. (2020), doi: 10.1158/2159-8290.CD-20-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frankel AE, Coughlin LA, Kim J, Froehlich TW, Xie Y, Frenkel EP, Koh AY, Metagenomic Shotgun Sequencing and Unbiased Metabolomic Profiling Identify Specific Human Gut Microbiota and Metabolites Associated with Immune Checkpoint Therapy Efficacy in Melanoma Patients. Neoplasia. 19, 848–855 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sender R, Fuchs S, Milo R, Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 14, e1002533 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belkaid Y, Naik S, Compartmentalized and systemic control of tissue immunity by commensals. Nat. Immunol 14, 646–653 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fung TC, Olson CA, Hsiao EY, Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci 20, 145–155 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zitvogel L, Ma Y, Raoult D, Kroemer G, Gajewski TF, The microbiome in cancer immunotherapy: Diagnostic tools and therapeutic strategies. Science. 359, 1366–1370 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Garrett WS, Cancer and the microbiota. Science. 348, 80–86 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gopalakrishnan V, Helmink BA, Spencer CN, Reuben A, Wargo JA, The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell. 33, 570–580 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Helmink BA, Khan MAW, Hermann A, Gopalakrishnan V, Wargo JA, The microbiome, cancer, and cancer therapy. Nat. Med 25, 377–388 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Parhi L, Alon-Maimon T, Sol A, Nejman D, Shhadeh A, Fainsod-Levi T, Yajuk O, Isaacson B, Abed J, Maalouf N, Nissan A, Sandbank J, Yehuda-Shnaidman E, Ponath F, Vogel J, Mandelboim O, Granot Z, Straussman R, Bachrach G, Breast cancer colonization by Fusobacterium nucleatum accelerates tumor growth and metastatic progression. Nat. Commun 11, 3259 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Locey KJ, Lennon JT, Scaling laws predict global microbial diversity. Proc. Natl. Acad. Sci. U. S. A 113, 5970–5975 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Biological agents. Volume 100 B. A review of human carcinogens. IARC Monogr. Eval. Carcinog. Risks Hum 100, 1–441 (2012). [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson MR, Jiang Y, Villalta PW, Stornetta A, Boudreau PD, Carrá A, Brennan CA, Chun E, Ngo L, Samson LD, Engelward BP, Garrett WS, Balbo S, Balskus EP, The human gut bacterial genotoxin colibactin alkylates DNA. Science. 363 (2019), doi: 10.1126/science.aar7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dejea CM, Fathi P, Craig JM, Boleij A, Taddese R, Geis AL, Wu X, DeStefano Shields CE, Hechenbleikner EM, Huso DL, Anders RA, Giardiello FM, Wick EC, Wang H, Wu S, Pardoll DM, Housseau F, Sears CL, Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science. 359, 592–597 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He Z, Gharaibeh RZ, Newsome RC, Pope JL, Dougherty MW, Tomkovich S, Pons B, Mirey G, Vignard J, Hendrixson DR, Jobin C, Campylobacter jejuni promotes colorectal tumorigenesis through the action of cytolethal distending toxin. Gut. 68, 289–300 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boot A, Ng AWT, Chong FT, Ho S-C, Yu W, Tan DSW, Iyer NG, Rozen SG, Characterization of colibactin-associated mutational signature in an Asian oral squamous cell carcinoma and in other mucosal tumor types. Genome Res. 30, 803–813 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barrett M, Hand CK, Shanahan F, Murphy T, O’Toole PW, Mutagenesis by Microbe: the Role of the Microbiota in Shaping the Cancer Genome. Trends Cancer Res. 6, 277–287 (2020). [DOI] [PubMed] [Google Scholar]
- 42.Silva-García O, Valdez-Alarcón JJ, Baizabal-Aguirre VM, Wnt/β-Catenin Signaling as a Molecular Target by Pathogenic Bacteria. Front. Immunol 10, 2135 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW, Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe. 14, 195–206 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kadosh E, Snir-Alkalay I, Venkatachalam A, May S, Lasry A, Elyada E, Zinger A, Shaham M, Vaalani G, Mernberger M, Stiewe T, Pikarsky E, Oren M, Ben-Neriah Y, The gut microbiome switches mutant p53 from tumour-suppressive to oncogenic. Nature. 586, 133–138 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, Agdashian D, Terabe M, Berzofsky JA, Fako V, Ritz T, Longerich T, Theriot CM, McCulloch JA, Roy S, Yuan W, Thovarai V, Sen SK, Ruchirawat M, Korangy F, Wang XW, Trinchieri G, Greten TF, Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 360 (2018), doi: 10.1126/science.aan5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Le Noci V, Guglielmetti S, Arioli S, Camisaschi C, Bianchi F, Sommariva M, Storti C, Triulzi T, Castelli C, Balsari A, Tagliabue E, Sfondrini L, Modulation of Pulmonary Microbiota by Antibiotic or Probiotic Aerosol Therapy: A Strategy to Promote Immunosurveillance against Lung Metastases. Cell Rep. 24, 3528–3538 (2018). [DOI] [PubMed] [Google Scholar]
- 47.Meisel M, Hinterleitner R, Pacis A, Chen L, Earley ZM, Mayassi T, Pierre JF, Ernest JD, Galipeau HJ, Thuille N, Bouziat R, Buscarlet M, Ringus DL, Wang Y, Li Y, Dinh V, Kim SM, McDonald BD, Zurenski MA, Musch MW, Furtado GC, Lira SA, Baier G, Chang EB, Eren AM, Weber CR, Busque L, Godley LA, Verdú EF, Barreiro LB, Jabri B, Microbial signals drive pre-leukaemic myeloproliferation in a Tet2-deficient host. Nature. 557, 580–584 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Almeida A, Mitchell AL, Boland M, Forster SC, Gloor GB, Tarkowska A, Lawley TD, Finn RD, A new genomic blueprint of the human gut microbiota. Nature. 568, 499–504 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dion MB, Oechslin F, Moineau S, Phage diversity, genomics and phylogeny. Nat. Rev. Microbiol 18, 125–138 (2020). [DOI] [PubMed] [Google Scholar]
- 50.Chassaing B, Kumar M, Baker MT, Singh V, Vijay-Kumar M, Mammalian gut immunity. Biomed. J 37, 246–258 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.The ICGC/TCGA Pan-Cancer Analysis of Whole Genomes Consortium, Pan-cancer analysis of whole genomes. Nature. 578 (2020), pp. 82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Del Monte U, Does the cell number 10(9) still really fit one gram of tumor tissue? Cell Cycle. 8, 505–506 (2009). [DOI] [PubMed] [Google Scholar]
- 53.Erdag G, Schaefer JT, Smolkin ME, Deacon DH, Shea SM, Dengel LT, Patterson JW, Slingluff CL Jr, Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res. 72, 1070–1080 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kather JN, Suarez-Carmona M, Charoentong P, Weis C-A, Hirsch D, Bankhead P, Horning M, Ferber D, Kel I, Herpel E, Schott S, Zörnig I, Utikal J, Marx A, Gaiser T, Brenner H, Chang-Claude J, Hoffmeister M, Jäger D, Halama N, Topography of cancer-associated immune cells in human solid tumors. Elife. 7 (2018), doi: 10.7554/eLife.36967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zitvogel L, Ayyoub M, Routy B, Kroemer G, Microbiome and Anticancer Immunosurveillance. Cell. 165, 276–287 (2016). [DOI] [PubMed] [Google Scholar]
- 56.Michelis FV, Messner HA, Loach D, Uhm J, Gupta V, Lipton JH, Seftel MD, Kuruvilla J, Kim DD, Early lymphocyte recovery at 28 d post-transplant is predictive of reduced risk of relapse in patients with acute myeloid leukemia transplanted with peripheral blood stem cell grafts. Eur. J. Haematol 93, 273–280 (2014). [DOI] [PubMed] [Google Scholar]
- 57.Staffas A, Burgos da Silva M, Slingerland AE, Lazrak A, Bare CJ, Holman CD, Docampo MD, Shono Y, Durham B, Pickard AJ, Cross JR, Stein-Thoeringer C, Velardi E, Tsai JJ, Jahn L, Jay H, Lieberman S, Smith OM, Pamer EG, Peled JU, Cohen DE, Jenq RR, van den Brink MRM, Nutritional Support from the Intestinal Microbiota Improves Hematopoietic Reconstitution after Bone Marrow Transplantation in Mice. Cell Host Microbe. 23, 447–457.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peled JU, Gomes ALC, Devlin SM, Littmann ER, Taur Y, Sung AD, Weber D, Hashimoto D, Slingerland AE, Slingerland JB, Maloy M, Clurman AG, Stein-Thoeringer CK, Markey KA, Docampo MD, Burgos da Silva M, Khan N, Gessner A, Messina JA, Romero K, Lew MV, Bush A, Bohannon L, Brereton DG, Fontana E, Amoretti LA, Wright RJ, Armijo GK, Shono Y, Sanchez-Escamilla M, Castillo Flores N, Alarcon Tomas A, Lin RJ, Yáñez San Segundo L, Shah GL, Cho C, Scordo M, Politikos I, Hayasaka K, Hasegawa Y, Gyurkocza B, Ponce DM, Barker JN, Perales M-A, Giralt SA, Jenq RR, Teshima T, Chao NJ, Holler E, Xavier JB, Pamer EG, van den Brink MRM, Microbiota as Predictor of Mortality in Allogeneic Hematopoietic-Cell Transplantation. N. Engl. J. Med 382, 822–834 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schluter J, Peled JU, Taylor BP, Markey KA, Smith M, Taur Y, Niehus R, Staffas A, Dai A, Fontana E, Amoretti LA, Wright RJ, Morjaria S, Fenelus M, Pessin MS, Chao NJ, Lew M, Bohannon L, Bush A, Sung AD, Hohl TM, Perales M-A, van den Brink MRM, Xavier JB, The gut microbiota is associated with immune cell dynamics in humans. Nature (2020), doi: 10.1038/s41586-020-2971-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brook I, Ledney GD, Effect of antimicrobial therapy on the gastrointestinal bacterial flora, infection and mortality in mice exposed to different doses of irradiation. J. Antimicrob. Chemother 33, 63–72 (1994). [DOI] [PubMed] [Google Scholar]
- 61.Burdelya LG, Krivokrysenko VI, Tallant TC, Strom E, Gleiberman AS, Gupta D, Kurnasov OV, Fort FL, Osterman AL, Didonato JA, Feinstein E, Gudkov AV, An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 320, 226–230 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu W, Chen Q, Wu S, Xia X, Wu A, Cui F, Gu Y-P, Zhang X, Cao J, Radioprotector WR-2721 and mitigating peptidoglycan synergistically promote mouse survival through the amelioration of intestinal and bone marrow damage. J. Radiat. Res 56, 278–286 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guo H, Chou W-C, Lai Y, Liang K, Tam JW, Brickey WJ, Chen L, Montgomery ND, Li X, Bohannon LM, Sung AD, Chao NJ, Peled JU, Gomes ALC, van den Brink MRM, French MJ, Macintyre AN, Sempowski GD, Tan X, Sartor RB, Lu K, Ting JPY, Multi-omics analyses of radiation survivors identify radioprotective microbes and metabolites. Science. 370 (2020), doi: 10.1126/science.aay9097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khosravi A, Yáñez A, Price JG, Chow A, Merad M, Goodridge HS, Mazmanian SK, Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe. 15, 374–381 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN, Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat. Med 16, 228–231 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fischer JC, Bscheider M, Eisenkolb G, Lin C-C, Wintges A, Otten V, Lindemans CA, Heidegger S, Rudelius M, Monette S, Porosnicu Rodriguez KA, Calafiore M, Liebermann S, Liu C, Lienenklaus S, Weiss S, Kalinke U, Ruland J, Peschel C, Shono Y, Docampo M, Velardi E, Jenq RR, Hanash AM, Dudakov JA, Haas T, van den Brink MRM, Poeck H, RIG-I/MAVS and STING signaling promote gut integrity during irradiation- and immune-mediated tissue injury. Sci. Transl. Med 9 (2017), doi: 10.1126/scitranslmed.aag2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Honda K, Littman DR, The microbiota in adaptive immune homeostasis and disease. Nature. 535, 75–84 (2016). [DOI] [PubMed] [Google Scholar]
- 68.Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillère R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ, Schlitzer A, Ginhoux F, Apetoh L, Chachaty E, Woerther P-L, Eberl G, Bérard M, Ecobichon C, Clermont D, Bizet C, Gaboriau-Routhiau V, Cerf-Bensussan N, Opolon P, Yessaad N, Vivier E, Ryffel B, Elson CO, Doré J, Kroemer G, Lepage P, Boneca IG, Ghiringhelli F, Zitvogel L, The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 342, 971–976 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Daillère R, Vétizou M, Waldschmitt N, Yamazaki T, Isnard C, Poirier-Colame V, Duong CPM, Flament C, Lepage P, Roberti MP, Routy B, Jacquelot N, Apetoh L, Becharef S, Rusakiewicz S, Langella P, Sokol H, Kroemer G, Enot D, Roux A, Eggermont A, Tartour E, Johannes L, Woerther P-L, Chachaty E, Soria J-C, Golden E, Formenti S, Plebanski M, Madondo M, Rosenstiel P, Raoult D, Cattoir V, Boneca IG, Chamaillard M, Zitvogel L, Enterococcus hirae and Barnesiella intestinihominis Facilitate Cyclophosphamide-Induced Therapeutic Immunomodulatory Effects. Immunity. 45, 931–943 (2016). [DOI] [PubMed] [Google Scholar]
- 70.Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CPM, Poirier-Colame V, Roux A, Becharef S, Formenti S, Golden E, Cording S, Eberl G, Schlitzer A, Ginhoux F, Mani S, Yamazaki T, Jacquelot N, Enot DP, Bérard M, Nigou J, Opolon P, Eggermont A, Woerther P-L, Chachaty E, Chaput N, Robert C, Mateus C, Kroemer G, Raoult D, Boneca IG, Carbonnel F, Chamaillard M, Zitvogel L, Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 350, 1079–1084 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre M-L, Chang EB, Gajewski TF, Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 350, 1084–1089 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Uribe-Herranz M, Rafail S, Beghi S, Gil-de-Gómez L, Verginadis I, Bittinger K, Pustylnikov S, Pierini S, Perales-Linares R, Blair IA, Mesaros CA, Snyder NW, Bushman F, Koumenis C, Facciabene A, Gut microbiota modulate dendritic cell antigen presentation and radiotherapy-induced antitumor immune response. J. Clin. Invest 130, 466–479 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bessell CA, Isser A, Havel JJ, Lee S, Bell DR, Hickey JW, Chaisawangwong W, Glick Bieler J, Srivastava R, Kuo F, Purohit T, Zhou R, Chan TA, Schneck JP, Commensal bacteria stimulate antitumor responses via T cell cross-reactivity. JCI Insight. 5 (2020), doi: 10.1172/jci.insight.135597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Paulos CM, Wrzesinski C, Kaiser A, Hinrichs CS, Chieppa M, Cassard L, Palmer DC, Boni A, Muranski P, Yu Z, Gattinoni L, Antony PA, Rosenberg SA, Restifo NP, Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J. Clin. Invest 117, 2197–2204 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roberti MP, Yonekura S, Duong CPM, Picard M, Ferrere G, Tidjani Alou M, Rauber C, Iebba V, Lehmann CHK, Amon L, Dudziak D, Derosa L, Routy B, Flament C, Richard C, Daillère R, Fluckiger A, Van Seuningen I, Chamaillard M, Vincent A, Kourula S, Opolon P, Ly P, Pizzato E, Becharef S, Paillet J, Klein C, Marliot F, Pietrantonio F, Benoist S, Scoazec J-Y, Dartigues P, Hollebecque A, Malka D, Pagès F, Galon J, Gomperts Boneca I, Lepage P, Ryffel B, Raoult D, Eggermont A, Vanden Berghe T, Ghiringhelli F, Vandenabeele P, Kroemer G, Zitvogel L, Chemotherapy-induced ileal crypt apoptosis and the ileal microbiome shape immunosurveillance and prognosis of proximal colon cancer. Nat. Med (2020), doi: 10.1038/s41591-020-0882-8. [DOI] [PubMed] [Google Scholar]
- 76.Shi Y, Zheng W, Yang K, Harris KG, Ni K, Xue L, Lin W, Chang EB, Weichselbaum RR, Fu Y-X, Intratumoral accumulation of gut microbiota facilitates CD47-based immunotherapy via STING signaling. J. Exp. Med 217 (2020), doi: 10.1084/jem.20192282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li Y, Tinoco R, Elmén L, Segota I, Xian Y, Fujita Y, Sahu A, Zarecki R, Marie K, Feng Y, Khateb A, Frederick DT, Ashkenazi SK, Kim H, Perez EG, Day C-P, Segura Muñoz RS, Schmaltz R, Yooseph S, Tam MA, Zhang T, Avitan-Hersh E, Tzur L, Roizman S, Boyango I, Bar-Sela G, Orian A, Kaufman RJ, Bosenberg M, Goding CR, Baaten B, Levesque MP, Dummer R, Brown K, Merlino G, Ruppin E, Flaherty K, Ramer-Tait A, Long T, Peterson SN, Bradley LM, Ronai ZA, Gut microbiota dependent anti-tumor immunity restricts melanoma growth in Rnf5−/− mice. Nat. Commun 10, 1492 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ansaldo E, Slayden LC, Ching KL, Koch MA, Wolf NK, Plichta DR, Brown EM, Graham DB, Xavier RJ, Moon JJ, Barton GM, Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis. Science. 364, 1179–1184 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gil-Cruz C, Perez-Shibayama C, De Martin A, Ronchi F, van der Borght K, Niederer R, Onder L, Lütge M, Novkovic M, Nindl V, Ramos G, Arnoldini M, Slack EMC, Boivin-Jahns V, Jahns R, Wyss M, Mooser C, Lambrecht BN, Maeder MT, Rickli H, Flatz L, Eriksson U, Geuking MB, McCoy KD, Ludewig B, Microbiota-derived peptide mimics drive lethal inflammatory cardiomyopathy. Science. 366, 881–886 (2019). [DOI] [PubMed] [Google Scholar]
- 80.Hebbandi Nanjundappa R, Ronchi F, Wang J, Clemente-Casares X, Yamanouchi J, Sokke Umeshappa C, Yang Y, Blanco J, Bassolas-Molina H, Salas A, Khan H, Slattery RM, Wyss M, Mooser C, Macpherson AJ, Sycuro LK, Serra P, McKay DM, McCoy KD, Santamaria P, A Gut Microbial Mimic that Hijacks Diabetogenic Autoreactivity to Suppress Colitis. Cell. 171, 655–667.e17 (2017). [DOI] [PubMed] [Google Scholar]
- 81.Petersen J, Ciacchi L, Tran MT, Loh KL, Kooy-Winkelaar Y, Croft NP, Hardy MY, Chen Z, McCluskey J, Anderson RP, Purcell AW, Tye-Din JA, Koning F, Reid HH, Rossjohn J, T cell receptor cross-reactivity between gliadin and bacterial peptides in celiac disease. Nat. Struct. Mol. Biol 27, 49–61 (2020). [DOI] [PubMed] [Google Scholar]
- 82.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, Hollmann TJ, Bruggeman C, Kannan K, Li Y, Elipenahli C, Liu C, Harbison CT, Wang L, Ribas A, Wolchok JD, Chan TA, Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med 371, 2189–2199 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fluckiger A, Daillère R, Sassi M, Sixt BS, Liu P, Loos F, Richard C, Rabu C, Alou MT, Goubet A-G, Lemaitre F, Ferrere G, Derosa L, Duong CPM, Messaoudene M, Gagné A, Joubert P, De Sordi L, Debarbieux L, Simon S, Scarlata C-M, Ayyoub M, Palermo B, Facciolo F, Boidot R, Wheeler R, Boneca IG, Sztupinszki Z, Papp K, Csabai I, Pasolli E, Segata N, Lopez-Otin C, Szallasi Z, Andre F, Iebba V, Quiniou V, Klatzmann D, Boukhalil J, Khelaifia S, Raoult D, Albiges L, Escudier B, Eggermont A, Mami-Chouaib F, Nistico P, Ghiringhelli F, Routy B, Labarrière N, Cattoir V, Kroemer G, Zitvogel L, Cross-reactivity between tumor MHC class I-restricted antigens and an enterococcal bacteriophage. Science. 369, 936–942 (2020). [DOI] [PubMed] [Google Scholar]
- 84.Tanoue T, Morita S, Plichta DR, Skelly AN, Suda W, Sugiura Y, Narushima S, Vlamakis H, Motoo I, Sugita K, Shiota A, Takeshita K, Yasuma-Mitobe K, Riethmacher D, Kaisho T, Norman JM, Mucida D, Suematsu M, Yaguchi T, Bucci V, Inoue T, Kawakami Y, Olle B, Roberts B, Hattori M, Xavier RJ, Atarashi K, Honda K, A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature. 565, 600–605 (2019). [DOI] [PubMed] [Google Scholar]
- 85.Coutzac C, Jouniaux J-M, Paci A, Schmidt J, Mallardo D, Seck A, Asvatourian V, Cassard L, Saulnier P, Lacroix L, Woerther P-L, Vozy A, Naigeon M, Nebot-Bral L, Desbois M, Simeone E, Mateus C, Boselli L, Grivel J, Soularue E, Lepage P, Carbonnel F, Ascierto PA, Robert C, Chaput N, Systemic short chain fatty acids limit antitumor effect of CTLA-4 blockade in hosts with cancer. Nat. Commun 11, 2168 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nomura M, Nagatomo R, Doi K, Shimizu J, Baba K, Saito T, Matsumoto S, Inoue K, Muto M, Association of Short-Chain Fatty Acids in the Gut Microbiome With Clinical Response to Treatment With Nivolumab or Pembrolizumab in Patients With Solid Cancer Tumors. JAMA Network Open. 3 (2020), p. e202895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li Y, Elmén L, Segota I, Xian Y, Tinoco R, Feng Y, Fujita Y, Segura Muñoz RR, Schmaltz R, Bradley LM, Ramer-Tait A, Zarecki R, Long T, Peterson SN, Ronai ZA, Prebiotic-Induced Anti-tumor Immunity Attenuates Tumor Growth. Cell Rep. 30, 1753–1766.e6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim OY, Park HT, Dinh NTH, Choi SJ, Lee J, Kim JH, Lee S-W, Gho YS, Bacterial outer membrane vesicles suppress tumor by interferon-γ-mediated antitumor response. Nat. Commun 8, 626 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, Lee JR, Offermanns S, Ganapathy V, Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 40, 128–139 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S, Dai R-M, Kiu H, Cardone M, Naik S, Patri AK, Wang E, Marincola FM, Frank KM, Belkaid Y, Trinchieri G, Goldszmid RS, Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 342, 967–970 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang L, Tang L, Feng Y, Zhao S, Han M, Zhang C, Yuan G, Zhu J, Cao S, Wu Q, Li L, Zhang Z, A purified membrane protein from Akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of CD8+ T cells in mice. Gut (2020), doi: 10.1136/gutjnl-2019-320105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vicente Dueñas C, Janssen S, Oldenburg M, Auer F, González Herrero I, Casado-García A, Isidro-Hernández M, Raboso-Gallego J, Westhoff P, Pandyra AA, Hein D, Gössling KL, Alonso-López D, De Las Rivas J, Bhatia S, Garcia-Criado FJ, García Cenador MB, Weber APM, Köhrer K, Hauer J, Fischer U, Sanchez-Garcia I, Borkhardt A, An intact gut microbiome protects genetically predisposed mice against leukemia. Blood (2020), doi: 10.1182/blood.2019004381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mager LF, Burkhard R, Pett N, Cooke NCA, Brown K, Ramay H, Paik S, Stagg J, Groves RA, Gallo M, Lewis IA, Geuking MB, McCoy KD, Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science. 369, 1481–1489 (2020). [DOI] [PubMed] [Google Scholar]
- 94.Amit M, Takahashi H, Dragomir MP, Lindemann A, Gleber-Netto FO, Pickering CR, Anfossi S, Osman AA, Cai Y, Wang R, Knutsen E, Shimizu M, Ivan C, Rao X, Wang J, Silverman DA, Tam S, Zhao M, Caulin C, Zinger A, Tasciotti E, Dougherty PM, El-Naggar A, Calin GA, Myers JN, Loss of p53 drives neuron reprogramming in head and neck cancer. Nature. 578, 449–454 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Venkataramani V, Tanev DI, Strahle C, Studier-Fischer A, Fankhauser L, Kessler T, Körber C, Kardorff M, Ratliff M, Xie R, Horstmann H, Messer M, Paik SP, Knabbe J, Sahm F, Kurz FT, Acikgöz AA, Herrmannsdörfer F, Agarwal A, Bergles DE, Chalmers A, Miletic H, Turcan S, Mawrin C, Hänggi D, Liu H-K, Wick W, Winkler F, Kuner T, Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature. 573, 532–538 (2019). [DOI] [PubMed] [Google Scholar]
- 96.Zeng Q, Michael IP, Zhang P, Saghafinia S, Knott G, Jiao W, McCabe BD, Galván JA, Robinson HPC, Zlobec I, Ciriello G, Hanahan D, Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature. 573, 526–531 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Venkatesh HS, Morishita W, Geraghty AC, Silverbush D, Gillespie SM, Arzt M, Tam LT, Espenel C, Ponnuswami A, Ni L, Woo PJ, Taylor KR, Agarwal A, Regev A, Brang D, Vogel H, Hervey-Jumper S, Bergles DE, Suvà ML, Malenka RC, Monje M, Electrical and synaptic integration of glioma into neural circuits. Nature. 573, 539–545 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Muller PA, Matheis F, Schneeberger M, Kerner Z, Jové V, Mucida D, Microbiota-modulated CART enteric neurons autonomously regulate blood glucose. Science. 370, 314–321 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Spanogiannopoulos P, Bess EN, Carmody RN, Turnbaugh PJ, The microbial pharmacists within us: a metagenomic view of xenobiotic metabolism. Nature Reviews Microbiology. 14 (2016), pp. 273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Daisley BA, Chanyi RM, Abdur-Rashid K, Al KF, Gibbons S, Chmiel JA, Wilcox H, Reid G, Anderson A, Dewar M, Nair SM, Chin J, Burton JP, Abiraterone acetate preferentially enriches for the gut commensal Akkermansia muciniphila in castrate-resistant prostate cancer patients. Nat. Commun 11, 4822 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mima K, Sukawa Y, Nishihara R, Qian ZR, Yamauchi M, Inamura K, Kim SA, Masuda A, Nowak JA, Nosho K, Kostic AD, Giannakis M, Watanabe H, Bullman S, Milner DA, Harris CC, Giovannucci E, Garraway LA, Freeman GJ, Dranoff G, Chan AT, Garrett WS, Huttenhower C, Fuchs CS, Ogino S, Fusobacterium nucleatum and T Cells in Colorectal Carcinoma. JAMA Oncol. 1, 653–661 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hamada T, Zhang X, Mima K, Bullman S, Sukawa Y, Nowak JA, Kosumi K, Masugi Y, Twombly TS, Cao Y, Song M, Liu L, da Silva A, Shi Y, Gu M, Li W, Koh H, Nosho K, Inamura K, Keum N, Wu K, Meyerhardt JA, Kostic AD, Huttenhower C, Garrett WS, Meyerson M, Giovannucci EL, Chan AT, Fuchs CS, Nishihara R, Giannakis M, Ogino S, Fusobacterium nucleatum in Colorectal Cancer Relates to Immune Response Differentially by Tumor Microsatellite Instability Status. Cancer Immunol Res. 6, 1327–1336 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Suzuki M, Mimuro H, Kiga K, Fukumatsu M, Ishijima N, Morikawa H, Nagai S, Koyasu S, Gilman RH, Kersulyte D, Berg DE, Sasakawa C, Helicobacter pylori CagA phosphorylation-independent function in epithelial proliferation and inflammation. Cell Host Microbe. 5, 23–34 (2009). [DOI] [PubMed] [Google Scholar]
- 104.Yu T, Guo F, Yu Y, Sun T, Ma D, Han J, Qian Y, Kryczek I, Sun D, Nagarsheth N, Chen Y, Chen H, Hong J, Zou W, Fang J-Y, Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell. 170, 548–563.e16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Forbes NS, Engineering the perfect (bacterial) cancer therapy. Nat. Rev. Cancer 10, 785–794 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhou S, Gravekamp C, Bermudes D, Liu K, Tumour-targeting bacteria engineered to fight cancer. Nat. Rev. Cancer 18, 727–743 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Garrett WS, Gordon JI, Glimcher LH, Homeostasis and inflammation in the intestine. Cell. 140, 859–870 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.O’Dwyer DN, Dickson RP, Moore BB, The Lung Microbiome, Immunity, and the Pathogenesis of Chronic Lung Disease. J. Immunol 196, 4839–4847 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stacy A, Belkaid Y, Microbial guardians of skin health. Science. 363, 227–228 (2019). [DOI] [PubMed] [Google Scholar]
- 110.Squarzanti DF, Zavattaro E, Pizzimenti S, Amoruso A, Savoia P, Azzimonti B, Non-Melanoma Skin Cancer: news from microbiota research. Crit. Rev. Microbiol 46, 433–449 (2020). [DOI] [PubMed] [Google Scholar]
- 111.Liu J, Luo M, Zhang Y, Cao G, Wang S, Association of high-risk human papillomavirus infection duration and cervical lesions with vaginal microbiota composition. Ann Transl Med. 8, 1161 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.So KA, Yang EJ, Kim NR, Hong SR, Lee J-H, Hwang C-S, Shim S-H, Lee SJ, Kim TJ, Changes of vaginal microbiota during cervical carcinogenesis in women with human papillomavirus infection. PLoS One. 15, e0238705 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Human Microbiome Project Consortium, Structure, function and diversity of the healthy human microbiome. Nature. 486, 207–214 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Campbell IM, Shaw CA, Stankiewicz P, Lupski JR, Somatic mosaicism: implications for disease and transmission genetics. Trends Genet. 31, 382–392 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bailey MH, Tokheim C, Porta-Pardo E, Sengupta S, Bertrand D, Weerasinghe A, Colaprico A, Wendl MC, Kim J, Reardon B, Ng PK-S, Jeong KJ, Cao S, Wang Z, Gao J, Gao Q, Wang F, Liu EM, Mularoni L, Rubio-Perez C, Nagarajan N, Cortés-Ciriano I, Zhou DC, Liang W-W, Hess JM, Yellapantula VD, Tamborero D, Gonzalez-Perez A, Suphavilai C, Ko JY, Khurana E, Park PJ, Van Allen EM, Liang H, MC3 Working Group, Cancer Genome Atlas Research Network, Lawrence MS, Godzik A, Lopez-Bigas N, Stuart J, Wheeler D, Getz G, Chen K, Lazar AJ, Mills GB, Karchin R, Ding L, Comprehensive Characterization of Cancer Driver Genes and Mutations. Cell. 173, 371–385.e18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Klein RS, Recco RA, Catalano MT, Edberg SC, Casey JI, Steigbigel NH, Association of Streptococcus bovis with carcinoma of the colon. N. Engl. J. Med 297, 800–802 (1977). [DOI] [PubMed] [Google Scholar]
- 117.Kwong TNY, Wang X, Nakatsu G, Chow TC, Tipoe T, Dai RZW, Tsoi KKK, Wong MCS, Tse G, Chan MTV, Chan FKL, Ng SC, Wu JCY, Wu WKK, Yu J, Sung JJY, Wong SH, Association Between Bacteremia From Specific Microbes and Subsequent Diagnosis of Colorectal Cancer. Gastroenterology. 155, 383–390.e8 (2018). [DOI] [PubMed] [Google Scholar]
- 118.Wirbel J, Pyl PT, Kartal E, Zych K, Kashani A, Milanese A, Fleck JS, Voigt AY, Palleja A, Ponnudurai R, Sunagawa S, Coelho LP, Schrotz-King P, Vogtmann E, Habermann N, Niméus E, Thomas AM, Manghi P, Gandini S, Serrano D, Mizutani S, Shiroma H, Shiba S, Shibata T, Yachida S, Yamada T, Waldron L, Naccarati A, Segata N, Sinha R, Ulrich CM, Brenner H, Arumugam M, Bork P, Zeller G, Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nat. Med 25, 679–689 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Thomas AM, Manghi P, Asnicar F, Pasolli E, Armanini F, Zolfo M, Beghini F, Manara S, Karcher N, Pozzi C, Gandini S, Serrano D, Tarallo S, Francavilla A, Gallo G, Trompetto M, Ferrero G, Mizutani S, Shiroma H, Shiba S, Shibata T, Yachida S, Yamada T, Wirbel J, Schrotz-King P, Ulrich CM, Brenner H, Arumugam M, Bork P, Zeller G, Cordero F, Dias-Neto E, Setubal JC, Tett A, Pardini B, Rescigno M, Waldron L, Naccarati A, Segata N, Metagenomic analysis of colorectal cancer datasets identifies cross-cohort microbial diagnostic signatures and a link with choline degradation. Nat. Med 25, 667–678 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yachida S, Mizutani S, Shiroma H, Shiba S, Nakajima T, Sakamoto T, Watanabe H, Masuda K, Nishimoto Y, Kubo M, Hosoda F, Rokutan H, Matsumoto M, Takamaru H, Yamada M, Matsuda T, Iwasaki M, Yamaji T, Yachida T, Soga T, Kurokawa K, Toyoda A, Ogura Y, Hayashi T, Hatakeyama M, Nakagama H, Saito Y, Fukuda S, Shibata T, Yamada T, Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nat. Med 25, 968–976 (2019). [DOI] [PubMed] [Google Scholar]
- 121.Flemer B, Lynch DB, Brown JMR, Jeffery IB, Ryan FJ, Claesson MJ, O’Riordain M, Shanahan F, O’Toole PW, Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut. 66, 633–643 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Farrell JJ, Zhang L, Zhou H, Chia D, Elashoff D, Akin D, Paster BJ, Joshipura K, Wong DTW, Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut. 61, 582–588 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Half E, Keren N, Reshef L, Dorfman T, Lachter I, Kluger Y, Reshef N, Knobler H, Maor Y, Stein A, Konikoff FM, Gophna U, Fecal microbiome signatures of pancreatic cancer patients. Sci. Rep 9, 16801 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yan X, Yang M, Liu J, Gao R, Hu J, Li J, Zhang L, Shi Y, Guo H, Cheng J, Razi M, Pang S, Yu X, Hu S, Discovery and validation of potential bacterial biomarkers for lung cancer. Am. J. Cancer Res 5, 3111–3122 (2015). [PMC free article] [PubMed] [Google Scholar]
- 125.Lee SH, Sung JY, Yong D, Chun J, Kim SY, Song JH, Chung KS, Kim EY, Jung JY, Kang YA, Kim YS, Kim SK, Chang J, Park MS, Characterization of microbiome in bronchoalveolar lavage fluid of patients with lung cancer comparing with benign mass like lesions. Lung Cancer. 102, 89–95 (2016). [DOI] [PubMed] [Google Scholar]
- 126.Greathouse KL, White JR, Vargas AJ, Bliskovsky VV, Beck JA, von Muhlinen N, Polley EC, Bowman ED, Khan MA, Robles AI, Cooks T, Ryan BM, Padgett N, Dzutsev AH, Trinchieri G, Pineda MA, Bilke S, Meltzer PS, Hokenstad AN, Stickrod TM, Walther-Antonio MR, Earl JP, Mell JC, Krol JE, Balashov SV, Bhat AS, Ehrlich GD, Valm A, Deming C, Conlan S, Oh J, Segre JA, Harris CC, Interaction between the microbiome and TP53 in human lung cancer. Genome Biol. 19, 123 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Urbaniak C, Cummins J, Brackstone M, Macklaim JM, Gloor GB, Baban CK, Scott L, O’Hanlon DM, Burton JP, Francis KP, Tangney M, Reid G, Microbiota of human breast tissue. Appl. Environ. Microbiol 80, 3007–3014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Urbaniak C, Gloor GB, Brackstone M, Scott L, Tangney M, Reid G, The Microbiota of Breast Tissue and Its Association with Breast Cancer. Appl. Environ. Microbiol 82, 5039–5048 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cantwell AR Jr, Kelso DW, Microbial findings in cancers of the breast and in their metastases to the skin. Implications for etiology. J. Dermatol. Surg. Oncol 7, 483–491 (1981). [DOI] [PubMed] [Google Scholar]
- 130.Gorelick JI, Senterfit LB, Vaughan ED Jr, Quantitative bacterial tissue cultures from 209 prostatectomy specimens: findings and implications. J. Urol 139, 57–60 (1988). [DOI] [PubMed] [Google Scholar]
- 131.Cohen RJ, Shannon BA, McNeal JE, Shannon T, Garrett KL, Propionibacterium acnes associated with inflammation in radical prostatectomy specimens: a possible link to cancer evolution? J. Urol 173, 1969–1974 (2005). [DOI] [PubMed] [Google Scholar]
- 132.Castellarin M, Warren RL, Freeman JD, Dreolini L, Krzywinski M, Strauss J, Barnes R, Watson P, Allen-Vercoe E, Moore RA, Holt RA, Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 22, 299–306 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pinato DJ, Howlett S, Ottaviani D, Urus H, Patel A, Mineo T, Brock C, Power D, Hatcher O, Falconer A, Ingle M, Brown A, Gujral D, Partridge S, Sarwar N, Gonzalez M, Bendle M, Lewanski C, Newsom-Davis T, Allara E, Bower M, Association of Prior Antibiotic Treatment With Survival and Response to Immune Checkpoint Inhibitor Therapy in Patients With Cancer. JAMA Oncol. 5, 1774–1778 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lowy DR, Schiller JT, Preventing Cancer and Other Diseases Caused by Human Papillomavirus Infection: 2017 Lasker-DeBakey Clinical Research Award. JAMA. 318, 901–902 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stolte M, Bayerdörffer E, Morgner A, Alpen B, Wündisch T, Thiede C, Neubauer A, Helicobacter and gastric MALT lymphoma. Gut. 50 Suppl 3, III19–24 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Huang X-Z, Gao P, Song Y-X, Xu Y, Sun J-X, Chen X-W, Zhao J-H, Wang Z-N, Antibiotic use and the efficacy of immune checkpoint inhibitors in cancer patients: a pooled analysis of 2740 cancer patients. Oncoimmunology. 8, e1665973 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Derosa L, Hellmann MD, Spaziano M, Halpenny D, Fidelle M, Rizvi H, Long N, Plodkowski AJ, Arbour KC, Chaft JE, Rouche JA, Zitvogel L, Zalcman G, Albiges L, Escudier B, Routy B, Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann. Oncol 29, 1437–1444 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Steck SE, Angela Murphy E, Dietary patterns and cancer risk. Nature Reviews Cancer. 20 (2020), pp. 125–138. [DOI] [PubMed] [Google Scholar]
- 139.Rad AH, Abbasi A, Kafil HS, Ganbarov K, Potential Pharmaceutical and Food Applications of Postbiotics: A review. Curr. Pharm. Biotechnol (2020), doi: 10.2174/1389201021666200516154833. [DOI] [PubMed] [Google Scholar]
- 140.van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JFWM, Tijssen JGP, Speelman P, Dijkgraaf MGW, Keller JJ, Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med 368, 407–415 (2013). [DOI] [PubMed] [Google Scholar]
- 141.Wang Y, Wiesnoski DH, Helmink BA, Gopalakrishnan V, Choi K, DuPont HL, Jiang Z-D, Abu-Sbeih H, Sanchez CA, Chang C-C, Parra ER, Francisco-Cruz A, Raju GS, Stroehlein JR, Campbell MT, Gao J, Subudhi SK, Maru DM, Blando JM, Lazar AJ, Allison JP, Sharma P, Tetzlaff MT, Wargo JA, Jenq RR, Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat. Med 24, 1804–1808 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.McQuade JL, Daniel CR, Helmink BA, Wargo JA, Modulating the microbiome to improve therapeutic response in cancer. Lancet Oncol 20, e77–e91 (2019). [DOI] [PubMed] [Google Scholar]
- 143.Baruch EN, Youngster I, Ben-Betzalel G, Ortenberg R, Lahat A, Katz L, Adler K, Dick-Necula D, Raskin S, Bloch N, Rotin D, Anafi L, Avivi C, Melnichenko J, Steinberg-Silman Y, Mamtani R, Harati H, Asher N, Shapira-Frommer R, Brosh-Nissimov T, Eshet Y, Ben-Simon S, Ziv O, Khan MAW, Amit M, Ajami NJ, Barshack I, Schachter J, Wargo JA, Koren O, Markel G, Boursi B, Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science (2020), doi: 10.1126/science.abb5920. [DOI] [PubMed] [Google Scholar]
- 144.Arthur JC, Gharaibeh RZ, Uronis JM, Perez-Chanona E, Sha W, Tomkovich S, Mühlbauer M, Fodor AA, Jobin C, VSL#3 probiotic modifies mucosal microbial composition but does not reduce colitis-associated colorectal cancer. Sci. Rep 3, 2868 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yelin I, Flett KB, Merakou C, Mehrotra P, Stam J, Snesrud E, Hinkle M, Lesho E, McGann P, McAdam AJ, Sandora TJ, Kishony R, Priebe GP, Genomic and epidemiological evidence of bacterial transmission from probiotic capsule to blood in ICU patients. Nat. Med 25, 1728–1732 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Conry RM, Westbrook B, McKee S, Norwood TG, Talimogene laherparepvec: First in class oncolytic virotherapy. Hum. Vaccin. Immunother 14, 839–846 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kramer MG, Masner M, Ferreira FA, Hoffman RM, Bacterial Therapy of Cancer: Promises, Limitations, and Insights for Future Directions. Front. Microbiol 9, 16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zheng M, Huang J, Tong A, Yang H, Oncolytic Viruses for Cancer Therapy: Barriers and Recent Advances. Mol Ther Oncolytics. 15, 234–247 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ylösmäki E, Cerullo V, Design and application of oncolytic viruses for cancer immunotherapy. Curr. Opin. Biotechnol 65, 25–36 (2019). [DOI] [PubMed] [Google Scholar]
- 150.Bommareddy PK, Shettigar M, Kaufman HL, Integrating oncolytic viruses in combination cancer immunotherapy. Nat. Rev. Immunol 18, 498–513 (2018). [DOI] [PubMed] [Google Scholar]
- 151.Charbonneau MR, Isabella VM, Li N, Kurtz CB, Developing a new class of engineered live bacterial therapeutics to treat human diseases. Nat. Commun 11, 1738 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zheng JH, Nguyen VH, Jiang S-N, Park S-H, Tan W, Hong SH, Shin MG, Chung I-J, Hong Y, Bom H-S, Choy HE, Lee SE, Rhee JH, Min J-J, Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin. Sci. Transl. Med 9 (2017), doi: 10.1126/scitranslmed.aak9537. [DOI] [PubMed] [Google Scholar]
- 153.Roberts NJ, Zhang L, Janku F, Collins A, Bai R-Y, Staedtke V, Rusk AW, Tung D, Miller M, Roix J, Khanna KV, Murthy R, Benjamin RS, Helgason T, Szvalb AD, Bird JE, Roy-Chowdhuri S, Zhang HH, Qiao Y, Karim B, McDaniel J, Elpiner A, Sahora A, Lachowicz J, Phillips B, Turner A, Klein MK, Post G, Diaz LA Jr, Riggins GJ, Papadopoulos N, Kinzler KW, Vogelstein B, Bettegowda C, Huso DL, Varterasian M, Saha S, Zhou S, Intratumoral injection of Clostridium novyi-NT spores induces antitumor responses. Sci. Transl. Med 6, 249ra111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Swofford CA, Van Dessel N, Forbes NS, Quorum-sensing Salmonella selectively trigger protein expression within tumors. Proc. Natl. Acad. Sci. U. S. A 112, 3457–3462 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Din MO, Danino T, Prindle A, Skalak M, Selimkhanov J, Allen K, Julio E, Atolia E, Tsimring LS, Bhatia SN, Hasty J, Synchronized cycles of bacterial lysis for in vivo delivery. Nature. 536, 81–85 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chowdhury S, Castro S, Coker C, Hinchliffe TE, Arpaia N, Danino T, Programmable bacteria induce durable tumor regression and systemic antitumor immunity. Nat. Med 25, 1057–1063 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Scott SR, Din MO, Bittihn P, Xiong L, Tsimring LS, Hasty J, A stabilized microbial ecosystem of self-limiting bacteria using synthetic quorum-regulated lysis. Nat Microbiol. 2, 17083 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Davenport ER, Sanders JG, Song SJ, Amato KR, Clark AG, Knight R, The human microbiome in evolution. BMC Biol. 15, 127 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Foster KR, Schluter J, Coyte KZ, Rakoff-Nahoum S, The evolution of the host microbiome as an ecosystem on a leash. Nature. 548, 43–51 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Janney A, Powrie F, Mann EH, Host–microbiota maladaptation in colorectal cancer. Nature. 585 (2020), pp. 509–517. [DOI] [PubMed] [Google Scholar]
- 161.Gharaibeh RZ, Jobin C, Microbiota and cancer immunotherapy: in search of microbial signals. Gut. 68, 385–388 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Limeta A, Ji B, Levin M, Gatto F, Nielsen J, Meta-analysis of the gut microbiota in predicting response to cancer immunotherapy in metastatic melanoma. JCI Insight. 5 (2020), doi: 10.1172/jci.insight.140940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sinha R, Abu-Ali G, Vogtmann E, Fodor AA, Ren B, Amir A, Schwager E, Crabtree J, Ma S, Microbiome Quality Control Project Consortium, Abnet CC, Knight R, White O, Huttenhower C, Assessment of variation in microbial community amplicon sequencing by the Microbiome Quality Control (MBQC) project consortium. Nat. Biotechnol 35, 1077–1086 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Eisenhofer R, Minich JJ, Marotz C, Cooper A, Knight R, Weyrich LS, Contamination in Low Microbial Biomass Microbiome Studies: Issues and Recommendations. Trends Microbiol. 27, 105–117 (2019). [DOI] [PubMed] [Google Scholar]
- 165.Lozupone CA, Stombaugh J, Gonzalez A, Ackermann G, Wendel D, Vázquez-Baeza Y, Jansson JK, Gordon JI, Knight R, Meta-analyses of studies of the human microbiota. Genome Res. 23, 1704–1714 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gonzalez A, Navas-Molina JA, Kosciolek T, McDonald D, Vázquez-Baeza Y, Ackermann G, DeReus J, Janssen S, Swafford AD, Orchanian SB, Sanders JG, Shorenstein J, Holste H, Petrus S, Robbins-Pianka A, Brislawn CJ, Wang M, Rideout JR, Bolyen E, Dillon M, Caporaso JG, Dorrestein PC, Knight R, Qiita: rapid, web-enabled microbiome meta-analysis. Nat. Methods 15, 796–798 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Davis NM, Proctor DM, Holmes SP, Relman DA, Callahan BJ, Simple statistical identification and removal of contaminant sequences in marker-gene and metagenomics data. Microbiome. 6, 226 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Merritt CR, Ong GT, Church SE, Barker K, Danaher P, Geiss G, Hoang M, Jung J, Liang Y, McKay-Fleisch J, Nguyen K, Norgaard Z, Sorg K, Sprague I, Warren C, Warren S, Webster PJ, Zhou Z, Zollinger DR, Dunaway DL, Mills GB, Beechem JM, Multiplex digital spatial profiling of proteins and RNA in fixed tissue. Nat. Biotechnol 38, 586–599 (2020). [DOI] [PubMed] [Google Scholar]
- 169.Shi H, Shi Q, Grodner B, Lenz JS, Zipfel WR, Brito IL, De Vlaminck I, Highly multiplexed spatial mapping of microbial communities. Nature (2020), doi: 10.1038/s41586-020-2983-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Min S, Kim S, Cho S-W, Gastrointestinal tract modeling using organoids engineered with cellular and microbiota niches. Exp. Mol. Med 52, 227–237 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Abed J, Emgård JEM, Zamir G, Faroja M, Almogy G, Grenov A, Sol A, Naor R, Pikarsky E, Atlan KA, Mellul A, Chaushu S, Manson AL, Earl AM, Ou N, Brennan CA, Garrett WS, Bachrach G, Fap2 Mediates Fusobacterium nucleatum Colorectal Adenocarcinoma Enrichment by Binding to Tumor-Expressed Gal-GalNAc. Cell Host Microbe. 20, 215–225 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Abed J, Maalouf N, Manson AL, Earl AM, Parhi L, Emgård JEM, Klutstein M, Tayeb S, Almogy G, Atlan KA, Chaushu S, Israeli E, Mandelboim O, Garrett WS, Bachrach G, Colon Cancer-Associated May Originate From the Oral Cavity and Reach Colon Tumors via the Circulatory System. Front. Cell. Infect. Microbiol 10, 400 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wassenaar TM, Insights from 100 Years of Research with Probiotic. Eur. J. Microbiol. Immunol 6, 147–161 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gurbatri CR, Lia I, Vincent R, Coker C, Castro S, Treuting PM, Hinchliffe TE, Arpaia N, Danino T, Engineered probiotics for local tumor delivery of checkpoint blockade nanobodies. Sci. Transl. Med 12 (2020), doi: 10.1126/scitranslmed.aax0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tanouchi Y, Smith RP, You L, Engineering microbial systems to explore ecological and evolutionary dynamics. Curr. Opin. Biotechnol 23, 791–797 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liao MJ, Din MO, Tsimring L, Hasty J, Rock-paper-scissors: Engineered population dynamics increase genetic stability. Science 365, 1045–1049 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lagier J-C, Dubourg G, Million M, Cadoret F, Bilen M, Fenollar F, Levasseur A, Rolain J-M, Fournier P-E, Raoult D, Culturing the human microbiota and culturomics. Nat. Rev. Microbiol 16, 540–550 (2018). [DOI] [PubMed] [Google Scholar]
- 178.Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang T-H, Porta-Pardo E, Gao GF, Plaisier CL, Eddy JA, Ziv E, Culhane AC, Paull EO, Sivakumar IKA, Gentles AJ, Malhotra R, Farshidfar F, Colaprico A, Parker JS, Mose LE, Vo NS, Liu J, Liu Y, Rader J, Dhankani V, Reynolds SM, Bowlby R, Califano A, Cherniack AD, Anastassiou D, Bedognetti D, Mokrab Y, Newman AM, Rao A, Chen K, Krasnitz A, Hu H, Malta TM, Noushmehr H, Pedamallu CS, Bullman S, Ojesina AI, Lamb A, Zhou W, Shen H, Choueiri TK, Weinstein JN, Guinney J, Saltz J, Holt RA, Rabkin CS, Cancer Genome Atlas Research Network, Lazar AJ, Serody JS, Demicco EG, Disis ML, Vincent BG, Shmulevich I, The Immune Landscape of Cancer. Immunity. 48, 812–830.e14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]


