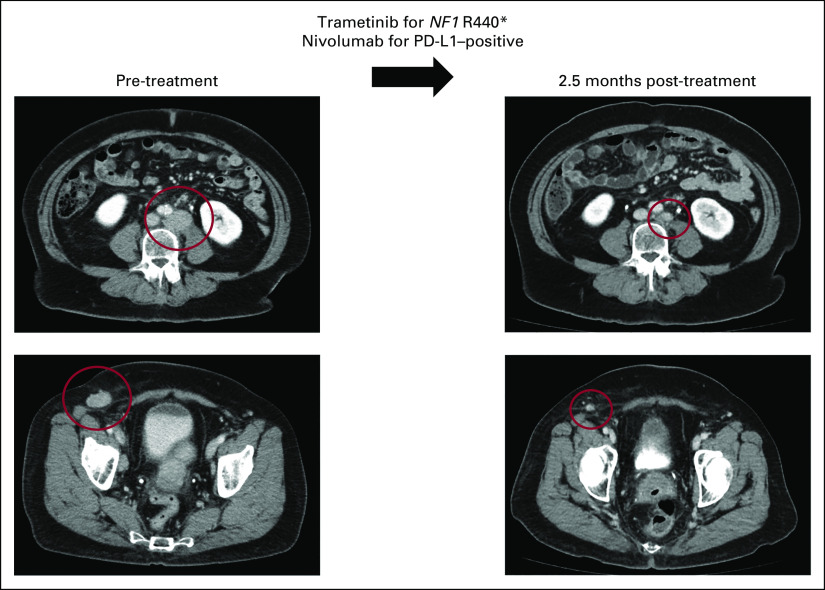
FIG 1.
Representative case of metastatic high-grade serous adenocarcinoma of likely gynecologic (favor tubo-ovarian) primary managed with matched targeted combination therapy approach. This is a 68-year-old woman who initially noticed enlargement of a right groin node (study ID: 4275). Biopsy was consistent with metastatic high-grade serous adenocarcinoma. CT showed multiple enlarged abdominal lymph nodes (left), with no obvious primary tumor on positron emission tomography. She was counseled on options for standard-of-care therapy with paclitaxel and carboplatin or enrollment in clinical trial. Tissue molecular profiling through Foundation Medicine showed alterations in NF1 R440*, CCND2 amplification, CCNE1 amplification, KDM5A amplification, and TP53 T231fs*9. Tumor mutation burden was low (3 mutations per megabase), microsatellite stable, and PD-L1 by immunohistochemistry showed low positivity (5%, Ventana SP142). The case was discussed at the Molecular Tumor Board with suggestion of trametinib (MEK inhibitor for NF1 R440*) and nivolumab (anti–programmed death 1 inhibitor for PD-L1 positivity). Patient also gave informed consent for an open-label navigational I-PREDICT study (ClinicalTrials.gov identifier: NCT02534675).7 The patient was started on trametinib 1 mg by mouth daily and nivolumab 240 mg intravenous every 2 weeks. CT 2.5 months after therapy was initiated showed partial response (left to right, best response 53% reduction by RECIST 1.1). Along with the reduction of tumor, tumor markers also normalized (CA153 before the therapy: 68.5 U/mL, nadir: 25 U/mL [normal range: 0-25 U/mL], CA125 before the therapy: 80 U/mL, nadir: 12 U/mL [normal range: 0-34 U/mL]). The patient tolerated the therapy well without major drug-related adverse events (experienced grade 1 rash). Treatment is ongoing for 19 months at the time of last follow-up. CA, cancer antigen; CT, computed tomography; I-PREDCIT, Investigation of molecular Profile-Related Evidence Determining Individualized Cancer Therapy for patients with aggressive malignancies; PD-L1, programmed death-ligand 1.