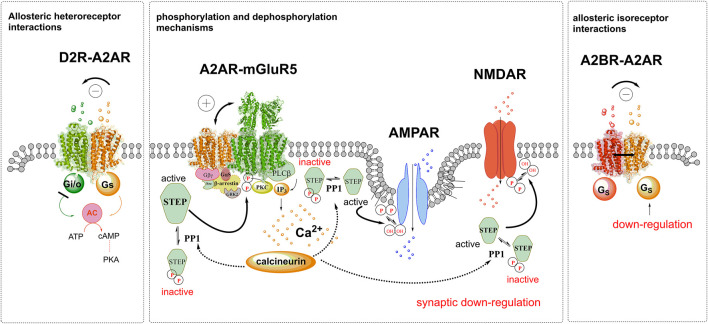
FIGURE 1.
Illustration of allosteric receptor-receptor interactions is shown in D2R-A2AR, A2AR-mGluR5 and A2BR-A2AR heterocomplexes and of activation of STEP through enhanced mGluR5 protomer signaling. To the left side, the A2AR agonist activation of the A2AR protomer leads to allosteric inhibition of the inhibitory Gi/o mediated signaling of the D2R, reducing the inhibition of the adenylate cyclase (AC). To the right side the constitutive activity (black line) of the A2BR leads to an allosteric downregulation of the Gs mediated activation of the A2AR. In the center the facilitatory allosteric interactions in the A2AR-mGluR5 heterocomplex is illustrated. It involves enhanced activity of the Gq mediated signaling of the mGluR5 with enhanced PLC-beta signaling leading to increased PKC activity with the enhanced formation IP3 increasing intracellular calcium levels. As a result, the calcineurin-PP1 pathway becomes activated and dephosphorylates STEP which causes its activation. The activated STEP can then dephosphorylate NMDAR and AMPAR with a return of the hydroxyl (OH) groups and a synaptic down regulation takes place. Also, mGluR5 binds STEP and becomes inactivated through its de-phosphorylation. In this way the mGluR5 may no longer be activated which becomes true also for Calcineurin and STEP activity becomes reduced with the return of synaptic activity.