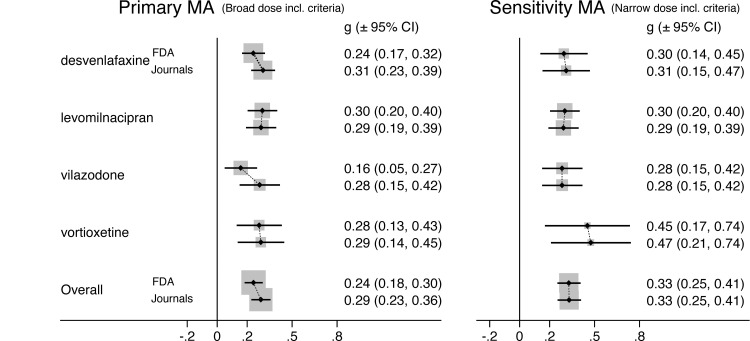
Fig 3. Meta-analytic ES of 4 newer antidepressants derived from trial reports in FDA reviews vs. journal articles.
The primary (left panel) and sensitivity (right panel) MAs were based on broad and narrow/restrictive dose inclusion criteria, respectively, as described in text. For each antidepressant, 2 drug-level ES values are shown, one based on clinical trial data from FDA reviews and one based on data from the journal articles. ES, effect size; g, Hedges’s g; FDA, Food and Drug Administration; MA, meta-analysis.