Abstract
We report a rare case of a basilar artery occlusion (BAO) caused by thrombosis as an initial magnification of acute myelogenous leukemia (AML) and performed mechanical thrombectomy (MT) to treat it. A 67-year-old female presented left hemiparalysis of her arm and right-sided blindness. Magnetic resonance imaging (MRI) and magnetic resonance angiography revealed acute infarction in the left occipital and anterior lobes of the cerebellum and incomplete BAO. Her blood test showed hyperleukocytosis with precursor cells and high levels of C-reactive protein, and we diagnosed AML and disseminated intravascular coagulation (DIC). We decided to treat conservatively with rapid rehydration and heparin, but three hours after admission, she suddenly lost consciousness. We performed acute MT with a direct aspiration first-pass technique (ADAPT). A white elastic embolus was aspirated, and DSA showed successful recanalization of the basilar artery. The next day, MRI revealed acute infarction in the midbrain and bilateral thalamus. The patient remained unconscious after MT and so chemotherapy to treat the acute leukemia could not be performed. The patient died of the primary disease 14 days after BAO. Thrombosis in association with AML is very rare disease and could occur in arterial vessels because of hypercoagulation, and this tendency may not respond to anticoagulation therapy. Although ADAPT might be performed safety without complications even in cases of DIC, indications for treatment with MT should be carefully considered in patients in whom hemorrhage is a possibility.
Keywords: endovascular mechanical thrombectomy, basilar artery occlusion, acute myelogenous leukemia, disseminated intravascular coagulation
Introduction
Acute myelogenous leukemia (AML), one of the most common hematologic malignancies, can lead to disseminated intravascular coagulation (DIC). Arterial thrombosis is a rare complication, and so effective treatment is not established. Consumption coagulopathy of DIC can lead to secondary bleeding.
Arterial large-vessel embolism resulting from AML is very rare, and no cases of basilar artery occlusion (BAO) have been reported until now. Because BAO is also a rare clinical entity, its endovascular surgical management has not been established.
Herein, we report a case of a combination of the two rare conditions in which acute BAO manifested as an initial symptom of AML and mechanical thrombectomy (MT) was performed as treatment without complications.
Case Presentation
A 67-year-old woman visited our emergency room with visual disturbance, fever, and fatigue, all of which had lasted for several days. She had undergone subtotal gastrectomy for stomach cancer 20 years earlier and endoscopic submucosal dissection for early colon cancer 3 years earlier. At the emergency room, her body temperature was 37.7°C, her blood pressure was 151/48 mmHg, and her Glasgow Coma Scale (GCS) score was 14 (E3V5M6). Her neurological examination revealed left hemiparalysis of her arm and right-sided blindness. Electrocardiographic study demonstrated sinus rhythm.
Routine blood tests performed immediately on admission revealed hyperleukocytosis with precursor cells, high levels of C-reactive protein, and DIC; the International Society on Thrombosis and Haemostasis algorithm yielded a score of 6 (Table 1).1)
Table 1. Laboratory examination.
| Chemistry | CBC | Coagulation | |||
|---|---|---|---|---|---|
| CRP | 16.3 mg/dl | WBC | 95.8 × 103/μl | PT | 14.0 sec |
| TP | 8.5 g/dl | Neut | 29.5% | INR | 1.21 |
| Alb | 3.1 g/dl | Lymph | 5.5% | APTT | 25.7 sec |
| AST | 20 U/L | Precursor cell | 64% | Fibrinogen | >700 mg/dl |
| ALT | 8 U/L | RBC | 2.51 × 106/μl | d-dimer | 17.9 μg/ml |
| LD | 515 U/L | Hb | 8.1 g/dl | ||
| BUN | 11 mg/dl | Hct | 25.8% | ||
| Cre | 0.7 mg/dl | MCV | 102.8 fL | ||
| Na | 137 mEq/L | MCH | 32.3 pg | ||
| K | 2 mEq/L | MCHC | 31.4% | ||
| Cl | 90 mEq/L | Plt | 98 × 103/μl | ||
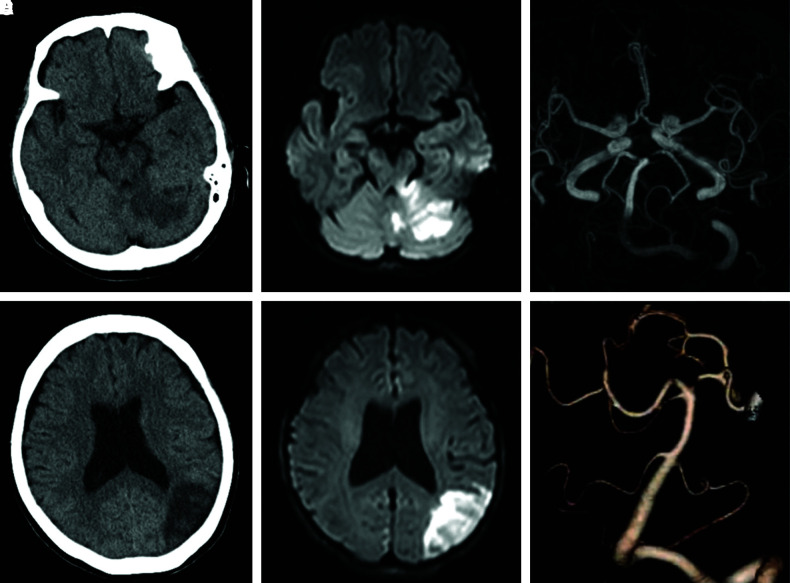
Head computed tomography (CT) without contrast medium revealed low-density areas in the left occipital and left anterior lobes of the cerebellum; the posterior circulation acute stroke prognosis early CT score (PC-ASPECTS) was 8. Magnetic resonance imaging (MRI) showed acute infarction in the left occipital and anterior lobes of the cerebellum (Fig. 1). Whole-body CT without contrast medium revealed no recurrence of previous cancers and no enlargement of lymph nodes.
Fig. 1. (A and B) Head CT on admission; there were low-density areas in left cerebellum and left occital-temporal lobe. (C and D) Diffusion-weighted-image on head MRI revealed high-intensity areas. (E) Basilar tip or left PCA occlusion was suspected on MRA. (F) After sudden unconsciousness, incomplete BAO was revealed on CTA. BAO: basilar artery occlusion, CT: computed tomography, CTA: computed tomography angiography, MRA: magnetic resonance angiography, MRI: magnetic resonance imaging, PCA: posterior cerebral artery, SCA: superior cerebellar artery.
The patient suddenly lost consciousness, and rapid intubation was performed without muscle relaxant. CT angiography with contrast medium showed incomplete basilar tip occlusion, and so we prepared to perform MT. However, she regained consciousness, up to a GCS score of 11 (E4VntM6), soon after CT angiography. Because she had regained consciousness, we suspected recanalization of BAO clinically; no established protocol to improve the prognosis of BAO existed, and so we decided to treat conservatively with rapid rehydration and heparin.
Three hours after admission, she suddenly lost consciousness again (GCS score of 2; E1VntM1). Head CT showed no hematoma or subarachnoid hemorrhage, so we suspected re-occlusion of the basilar artery and performed digital subtraction angiography (DSA), which revealed thrombosis of the basilar artery tip and occlusion of the left posterior cerebral artery. Acute MT with a direct aspiration first-pass technique (ADAPT) were performed. A guiding catheter (7F ENVOY STR; Johnson & Johnson, Miami, FL, USA) was placed in the left vertebral artery. Under road-map guidance, a 0.014-inch microwire (Synchro2 standard; Stryker Corporation, Fremont, CA, USA) and a microcatheter (Excelsior SL-10; Stryker Corporation) were navigated past the thrombosis to the right posterior cerebral artery. Then, the aspiration catheter (5F SOFIA Flow Plus; MicroVention, Aliso Viejo, CA, USA) was positioned immediately adjacent to the site of occlusion. The microwire and microcatheter were removed, and aspiration was performed through an aspiration syringe and withdrawn into the guiding catheter (Fig. 2).
Fig. 2. (A) Preoperative left vertebral artery angiography (anterior–posterior view) revealed the basilar tip occlusion and left PCA was not contrasted. (B) We withdrawn SOFIAFLOW into the guiding catheter (ENVOY) (ADAPT technique). (C) Successful recanalization of basilar artery was obtained after thrombectomy (anterior–posterior view). (D–G) Postoperative head MRI and CT revealed new infarction in bilateral midbrain and thalamus medialis. ADAPT: a direct aspiration first-pass technique, CT: computed tomography, MRI: magnetic resonance imaging, PCA: posterior cerebral artery.
In the first aspiration, a white elastic embolus was aspirated, and DSA showed successful recanalization of the basilar artery. The time between diagnosis to recanalization was 42 minutes. DSA revealed a remaining thrombus, however, and a second ADAPT was performed. The second thrombus was similar to the first one. These obtained thrombi were mainly composed of fibrin but also contained leukemic blasts (Fig. 3A and 3B). The next day, MRI revealed high-intensity areas in the midbrain and bilateral thalamus, and infarctions of perforating arteries (i.e., posterior thalamic perforating arteries) from the basilar artery tip (Fig. 2). The patient remained unconscious after MT, and so chemotherapy to treat the acute leukemia could not be performed. The patient died of the primary disease 14 days after BAO. We could not obtain her family’s consent to pathological autopsy.
Fig. 3. (A and B) Hematoxylin and eosin stain of obtained emboli showed the emboli constituted mainly with fibrin. Interestingly, the thrombi contained many deformed blast cells. (C) Bone marrow revealed over 99% positive cells with peroxidase stain.
Bone marrow aspirates and biopsy samples revealed increased abnormal leukemic cells, of which 99% yielded positive findings with peroxidase stain. Giemsa banding showed normal chromosomes, and no specific mutation was found on genetic testing. Therefore, hematologists diagnosed as AML not otherwise specified without mutation according to the World Health Organization’s classification of AML and as M1 according to the French–American–British classification of AML (Fig. 3).
Discussion
Our report concerns a rare disease entity, BAO as an initial manifestation of AML, and a rare treatment for it: MT. Data concerning the risk of thrombosis in AML were scarce.
AML is known to lead to DIC. In DIC, significant and persistent systemic activation of coagulation results in multiple microthrombi, mainly in small vessels, throughout the body and decreases in platelet and coagulation factors. DIC associated with AML is thought to be caused by the activation of extrinsic coagulation by tumor cell-derived tissue factor, and the expression of tissue factor on monocytes or macrophages and vascular endothelial cells can result in multiple fibrin clots throughout the body. In addition, plasminogen activator released from tumor cells is known to activate fibrinolysis excessively and lead to hyperfibrinolytic DIC.2)
Previous reports have indicated that 6.3% of patients with AML have thrombosis, which is arterial in 20% of those cases.3) Because of the rarity of AML-associated thrombosis, most usable data are found in some case reports or small retrospective studies.
Trousseau syndrome is another stroke type, caused by malignancy-associated hypercoagulable situation or DIC.4) Most malignant cancers that predispose patients to stroke have been reported to be adenocarcinomas, such as gynecologic tumors, renal-genitourinary tract tumors, and gastrointestinal tumors.5) Tissue factor or mucin secreted from solid tumors, such as ovarian cancer, may lead to hypercoagulation and then to Trousseau syndrome. Because precursor cells of AML secrete tissue factor, thrombosis in AML may be caused by a mechanism of hypercoagulation similar to that underlying the Trousseau syndrome.
Large arterial thromboses attributable to AML are very rare. They have been reported in the carotid, iliofemoral, and coronary arteries.6–8) Some have been treated successfully with surgery or endovascular treatment, but invasive management is highly risky. When symptoms of occlusion are mild, anticoagulation is a standard conservative treatment of normal thrombosis to prevent re-occlusion, but it may not be effective against thrombosis induced by AML, according to past reports.9) The pathological findings in our patient showed numerous blast cells contained in the clots, and thrombosis may have recurred locally around the clot because the blast cells may have secreted tissue factor. The lack of stability of thrombosis that led to re-occlusion may have worsened the prognosis.
BAO is very rare, accounting for only 1% of all strokes; it carries a high risk of mortality and disability.10–12) Endovascular treatment enables surgeons to treat acute ischemic stroke, after which MT is an established treatment for acute major trunk embolism, especially internal carotid artery occlusion and proximal middle cerebral artery occlusion. However, although MT for BAO appears to be effective, no current evidence indicates that it improves neurological prognosis. According to some reports, the incidence of postoperative symptomatic hemorrhage was significantly higher after MT than after medical therapy.13) The efficiency of MT is suggested to depend on PC-ASPECTS or shorter time to successful recanalization.14–16) Our patient had high PC-ASPECTS and obtained rapid recanalization, but her poor outcome was attributable to infarction of the perforating arteries.17,18)
In some cases in which MT was performed, major artery occlusion was caused by cancer-associated cerebral stroke, solid tumors (e.g., lung cancer), or cardiac tumors (e.g., cardiac myxoma).19) In two of these cases, MT was performed for BAO that was induced by gastric cancer and lung tumor.
ADAPT is an established method of MT and is considered safer than the use of stent retrievers. Histological changes caused by stent-retrieving thrombectomy may induce vascular damages that extend to the medial layer,20) giving rise to postoperative hemorrhagic complications.
No standard management for thrombosis that is associated with AML has been established, and in our patient, management of BAO was particularly challenging. Although MT carries a very high risk for complications, such as easy bleeding and easy embolization, ADAPT enabled us to perform this procedure without complications. In patients with AML, the risk of fatal intracranial hemorrhage is high because of the tendency for coagulopathy in this disease.21–23) MT could lead to fatal complications, such as severe intracranial hemorrhage. Easy hemorrhage and hypercoagulation are contraindications to invasive surgery. ADAPT may be useful in such situations.
Conclusion
Our patient had a rare condition, BAO as an early manifestation of AML, and underwent a rare treatment, MT. Thrombosis in association with AML could occur in arterial vessels because of hypercoagulation, and this tendency may not respond to anticoagulation therapy. Although ADAPT might be performed safely without complications even in cases of DIC, indications for treatment with MT should be carefully considered in patients in whom hemorrhage is a possibility.
Footnotes
Conflicts of Interest Disclosure
The authors have no personal financial or institutional interests in any of the drugs, materials, or devices in the article. The authors who are members of the Japan Neurosurgical Society (JNS) have registered online Self-reported COI Disclosure Statement Forms through the website for JNS members.
References
- 1). Taylor FB, Toh CH, Hoots WK, Wada H, Levi M: Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH): Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost 86: 1327– 1330, 2001 [PubMed] [Google Scholar]
- 2). Kawasugi K, Wada H, Hatada T, et al. : Prospective evaluation of hemostatic abnormalities in overt DIC due to various underlying diseases. Thromb Res 128: 186– 190, 2011 [DOI] [PubMed] [Google Scholar]
- 3). De Stefano V, Sorà F, Rossi E, et al. : The risk of thrombosis in patients with acute leukemia: occurrence of thrombosis at diagnosis and during treatment. J Thromb Haemost 3: 1985– 1992, 2005 [DOI] [PubMed] [Google Scholar]
- 4). Varki A: Trousseau’s syndrome: multiple definitions and multiple mechanisms. Blood 110: 1723– 1729, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Chaturvedi S, Ansell J, Recht L: Should cerebral ischemic events in cancer patients be considered a manifestation of hypercoagulability? Stroke 25: 1215– 1218, 1994 [DOI] [PubMed] [Google Scholar]
- 6). Saitoh E, Sugita K, Kurosawa H, et al. : Cerebral infarction in acute promyelocytic leukemia at initial presentation. Acta Paediatr Jpn 37: 710– 712, 1995 [DOI] [PubMed] [Google Scholar]
- 7). Chavez MA, Heidari B, Thacker S, Samuel LL, Ogbonna M: Acute promyelocytic leukemia presenting as bilateral acute limb ischemia and ST elevation myocardial infarction: a case report. Cureus 12: e8495, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Fass R, Haddad M, Zaizov R, et al. : Recurrent peripheral arterial occlusion by leukemic cells sedimentation in acute promyelocytic leukemia. J Pediatr Surg 27: 665– 667, 1992 [DOI] [PubMed] [Google Scholar]
- 9). Kafetzakis A, Foundoulakis A, Ioannou CV, Stavroulaki E, Koutsopoulos A, Katsamouris AN: Acute lower limb ischemia as the initial symptom of acute myeloid leukemia. Vasc Med 12: 199– 202, 2007 [DOI] [PubMed] [Google Scholar]
- 10). Singer OC, Berkefeld J, Nolte CH, et al. : Mechanical recanalization in basilar artery occlusion: the ENDOSTROKE study. Ann Neurol 77: 415– 424, 2015 [DOI] [PubMed] [Google Scholar]
- 11). Mattle HP, Arnold M, Lindsberg PJ, Schonewille WJ, Schroth G: Basilar artery occlusion. Lancet Neurol 10: 1002– 1014, 2011 [DOI] [PubMed] [Google Scholar]
- 12). Schonewille WJ, Wijman CA, Michel P, et al. : Treatment and outcomes of acute basilar artery occlusion in the Basilar Artery International Cooperation Study (BASICS): a prospective registry study. Lancet Neurol 8: 724– 730, 2009 [DOI] [PubMed] [Google Scholar]
- 13). Fisher M: Endovascular therapy for basilar-artery occlusion — still waiting for answers. N Engl J Med 384: 1954– 1955, 2021 [DOI] [PubMed] [Google Scholar]
- 14). Kwak HS, Park JS: Mechanical thrombectomy in basilar artery occlusion: clinical outcomes related to posterior circulation collateral score. Stroke 51: 2045– 2050, 2020 [DOI] [PubMed] [Google Scholar]
- 15). Meinel TR, Kaesmacher J, Chaloulos-Iakovidis P, et al. : Mechanical thrombectomy for basilar artery occlusion: efficacy, outcomes, and futile recanalization in comparison with the anterior circulation. J Neurointerv Surg 11: 1174– 1180, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Lindsberg PJ, Mattle HP: Therapy of basilar artery occlusion: a systematic analysis comparing intra-arterial and intravenous thrombolysis. Stroke 37: 922– 928, 2006 [DOI] [PubMed] [Google Scholar]
- 17). Nishi A, Goto Y, Yamanaka K, Kishima H: A direct aspiration first pass technique for basilar artery occlusion caused by elastic-hard tumor embolus via the pulmonary vein by metastatic prostate adenocarcinoma: a case report. NMC Case Rep J 8: 95– 100, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Nishimuta Y, Nagayama T, Hiwatari T, et al. : A patient with cerebral embolism related to Trousseau’s syndrome. JNET J Neuroendovasc Ther 11: 575– 580, 2017 [Google Scholar]
- 19). Tsurusaki Y, Takahara K, Koga N, Amano T, Haga S, Arihiro S: A case of mechanical reperfusion therapy for cerebral infarction induced by tumor embolism from lung cancer. JNET J Neuroendovasc Ther 13: 342– 347, 2019 [Google Scholar]
- 20). Arai D, Ishii A, Chihara H, Ikeda H, Miyamoto S: Histological examination of vascular damage caused by stent retriever thrombectomy devices. J Neurointerv Surg 8: 992– 995, 2016 [DOI] [PubMed] [Google Scholar]
- 21). Chen CY, Tai CH, Tsay W, Chen PY, Tien HF: Prediction of fatal intracranial hemorrhage in patients with acute myeloid leukemia. Ann Oncol 20: 1100– 1104, 2009 [DOI] [PubMed] [Google Scholar]
- 22). Posacioglu H, Apaydin AZ, Buyukkececi F, Soydan S, Durmaz I.: Recurrent peripheral arterial occlusion in acute promyelocytic leukemia. EJVES Extra 6: 100– 102, 2003 [Google Scholar]
- 23). Carnevale ML, Phair J, Yau P, Garg K: Blast cell arterial embolus in acute myelogenous leukemia. Ann Vasc Surg 56: 351.e9– 351.e11, 2019 [DOI] [PubMed] [Google Scholar]