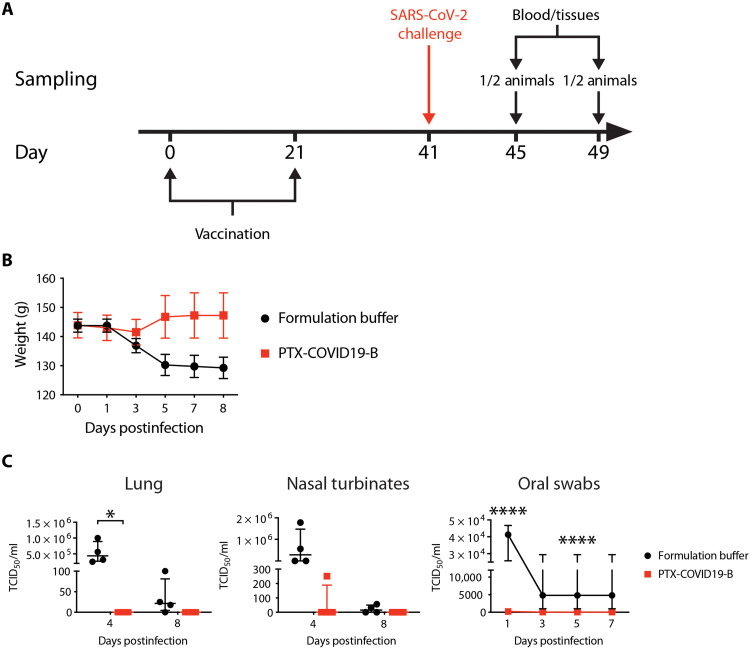
Fig. 6. PTX-COVID19-B protects hamsters from SARS-CoV-2 challenge.
(A) Hamster vaccination and SARS-CoV-2 challenge regimen. Six- to 10-week-old male Syrian hamsters (n = 8) were vaccinated with a 20-μg dose of PTX-COVID19-B or formulation buffer twice with a 3-week interval. Twenty days after the second vaccination, hamsters were challenged intranasally with SARS-CoV-2. Four and 8 days after the challenge, half of the animals in each group (four animals per group) were humanely euthanized, and blood and tissues were collected. (B) Body weight of the hamsters. Symbols indicate the means, and the error bars indicate the SEM for each group. N = 8 per group from days 0 to 3. N = 4 from days 5 to 8. (C) Amount of infectious SARS-CoV-2 virus in the hamster tissues. Shown are TCID50 values per milliliter of the tissue homogenates (lung and nasal turbinates) or per milliliter of oral swab samples. For lungs and nasal turbinates, each symbol represents one hamster (n = 4 per group per time point), the long horizontal line indicates the median, and the short lines below and above the median indicate the 25th and 75th percentiles. For oral swabs, symbols indicate the medians, and the error bars indicate the 25th and 75th percentiles (n = 8 per group from days 0 to 3 and n = 4 from days 5 to 7). *P < 0.05 and ****P < 0.0001 as determined by two-way ANOVA followed by Sidak’s multiple comparison test.