Figure 4. Genome editing-induced disruption of p110γPI3K expression in pulmonary vascular ECs resulted in impaired vascular repair and resolution of inflammation as seen in Pik3cg−/− mice.
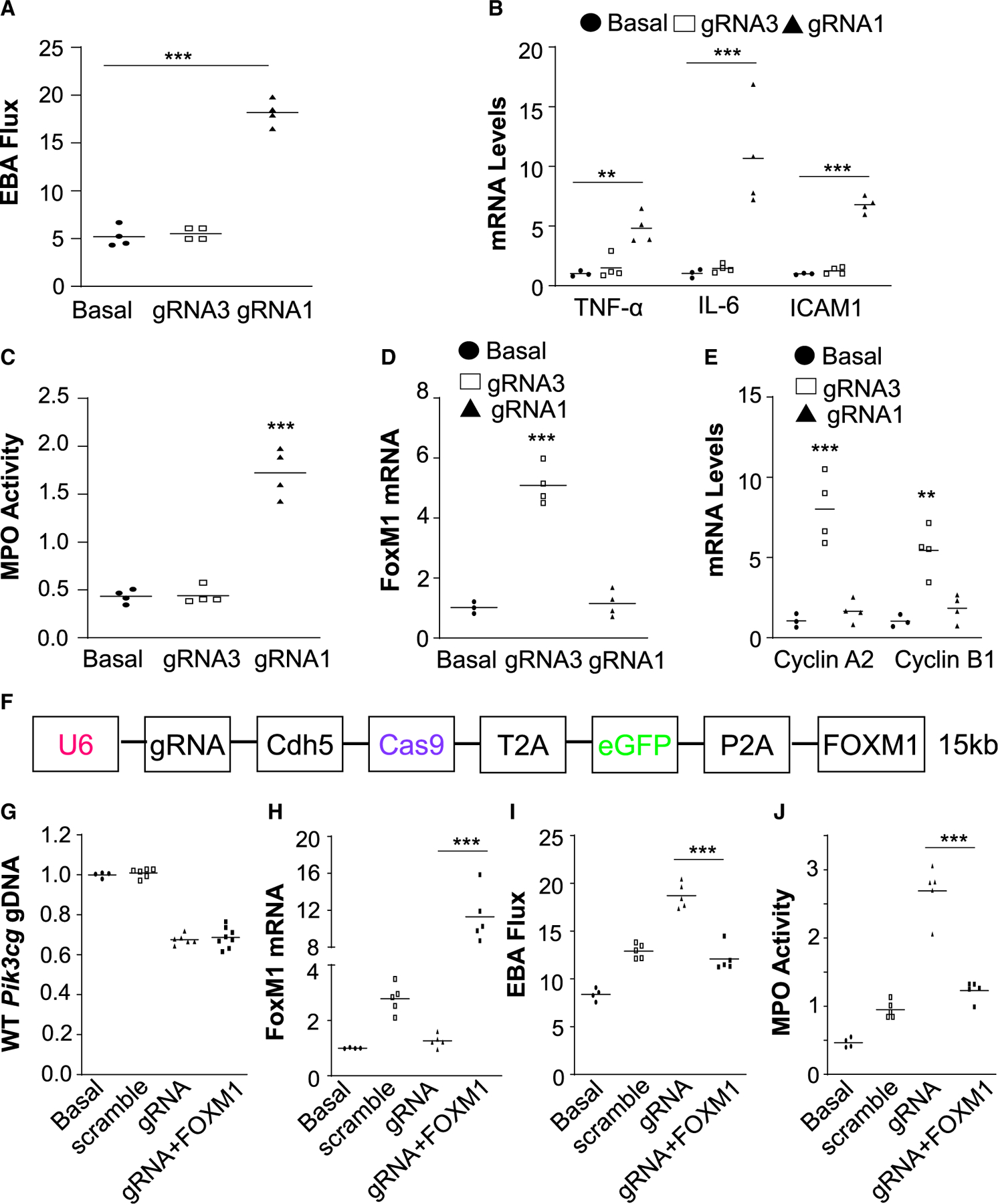
(A) Measurement of pulmonary transvascular EBA flux demonstrating defective vascular repair in gRNA1 nanoparticle-treated mice in contrast to gRNA3 nanoparticle-treated mice. Seven days post-nanoparticle:DNA administration, the mice were challenged with LPS. At 72 h post-LPS, lung tissues were collected for analyses (n = 4). Basal, WT mice without administration of nanoparticle/DNA and LPS challenge.
(B) QRT-PCR analysis demonstrating marked increases of expression of proinflammatory genes in lungs of gRNA1 nanoparticle-treated mice (n = 3 or 4).
(C) Persistently elevated lung myeloperoxidase (MPO) activity in gRNA1 nanoparticle-treated mice (n = 4).
(D and E) QRT-PCR analysis showing inhibited expression of the transcription factor FoxM1 (D) and its target genes (E) in lungs of gRNA1 nanoparticle-treated mice (n = 3 or 4).
(F) Diagram showing a large CRISPRCDH5 plasmid expressing a 3-kb FOXM1 transgene. (G) Similar genome editing efficiency in lung ECs by different sizes of CRISPR plasmid. Four days post-administration of PP/PEI nanoparticles:plasmid DNA complexes (40 μg/mouse), the mice were challenged with LPS and lung tissues were collected at 72 h post-LPS for various analyses (n = 4 or 6).
(H) QRT-PCR analysis showing 12-fold induction of FoxM1 expression in lungs of mice with the CRISPRCDH5-FOXM1 plasmid (n = 4 or 5).
(I and J) FOXM1 transgene expression recused the defective vascular repair phenotype induced by gRNA-mediated knockout of p110γPI3K, which is the upstream mediator (n = 4 or 5). **p < 0.01, ***p < 0.001. One-way ANOVA with Bonferroni post hoc analysis (A, C, D); Student’s t test (B, E, H, I, J).
