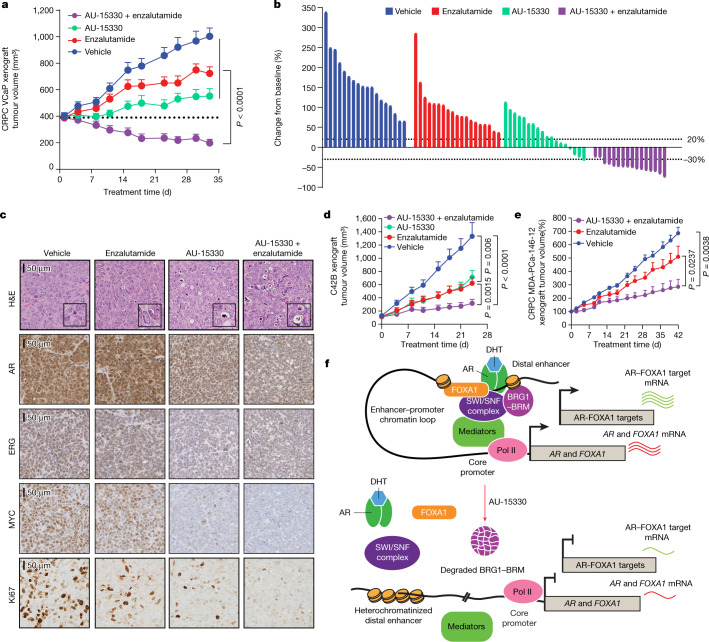
Fig. 4. AU-15330 inhibits tumour growth in preclinical models of CRPC and synergizes with enzalutamide.
a, Tumour volume (measured twice per week using callipers) in the VCaP-CRPC model with AU-15330 alone or in combination with enzalutamide (two-sided t-test). Data are mean ±s.e.m. (vehicle: n = 18; AU-15330: n = 20; enzalutamide: n = 18; AU-15330 + enzalutamide: n = 16). b, Waterfall plot depicting change in tumour volume after 33 days of treatment. Response evaluation criteria in solid tumours (RECIST) was used to stratify tumours: progressive disease (PD), at least a 20% increase in tumour size; stable disease (SD), increase of <20% to a decrease of <30%; partial response (PR), at least a 30% decrease. The vehicle and enzalutamide groups have 100% PD; the AU-15330 group has 61% PD, 33% SD and 6% PR; and the AU-15330 + enzalutamide group has 0% PD, 12% SD and 88% PR. c, Representative haematoxylin and eosin (H&E) staining and immunohistochemistry from the VCaP-CRPC xenograft study (n = 2 tumours per condition). Insets in the H&E images show expanded views of apoptotic cells. d, Tumour volume measurements showing efficacy of AU-15330, enzalutamide or combined treatment in C4-2B-derived CRPC xenografts (n = 20 per condition; two-sided t-test). Data are mean ± s.e.m. e, Tumour volume measurements showing the effect of enzalutamide alone or in combination with AU-15330 in the castration-resistant MDA-PCa-146-12 PDX study (two-sided t-test). Data are mean ± s.e.m. f, Mechanism of action of AU-15330-triggered cytotoxicity in AR–FOXA1-signalling-driven prostate cancer. SWI/SNF ATPase degradation induces a rapid, targeted loss in chromatin accessibility at the core-enhancer circuitry of AR, FOXA1, ERG and MYC, thereby attenuating their cancer-promoting transcriptional programs and tempering the enhancer-wired supra-physiologic expression of driver oncogenes.