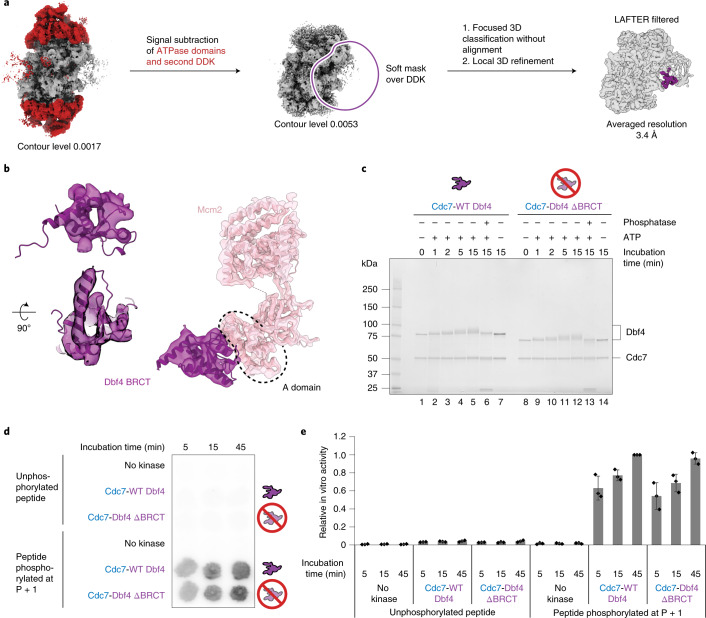
Fig. 3. The Dbf4 BRCT domain docks onto the A domain of Mcm2 and its truncation does not affect Cdc7 catalytic activity.
a, Signal subtraction of the MCM ATPase domains, followed by focused classification, 3D refinement and LAFTER filtering, allowed resolution of the docking site of DDK (shown in purple) onto the MCM DH. b, The crystal structure of the Dbf4 BRCT domain (PDB entry 3QBZ) was fitted to the newly resolved docking site, which contacts the A domain of the Mcm2 subunit. c, Wild-type (WT) DDK (lanes 1–7) and DDK containing a Dbf4 BRCT truncation (lacking residues 119–219, lanes 8–14) show comparable autophosphorylation efficiency, which can be reverted by lambda phosphatase treatment (lanes 7 and 14). Representative of at least three independent experiments. d, Autoradiograph of a kinase assay using a well-characterized substrate of DDK kinases (residues 35–47 of human Mcm2). e, Quantification of the kinase assay. The average of three biological replicas is plotted and error bars show s.d. Reads were normalized to the 45-min time point of wild-type DDK. The DDK mutant shows wild-type levels of MCM phosphorylation. As described for the human ortholog, phosphorylation by Cdc7 requires prephosphorylation of Ser41 (P + 1). Uncropped gel images for c and d are available as Source data with the paper online.