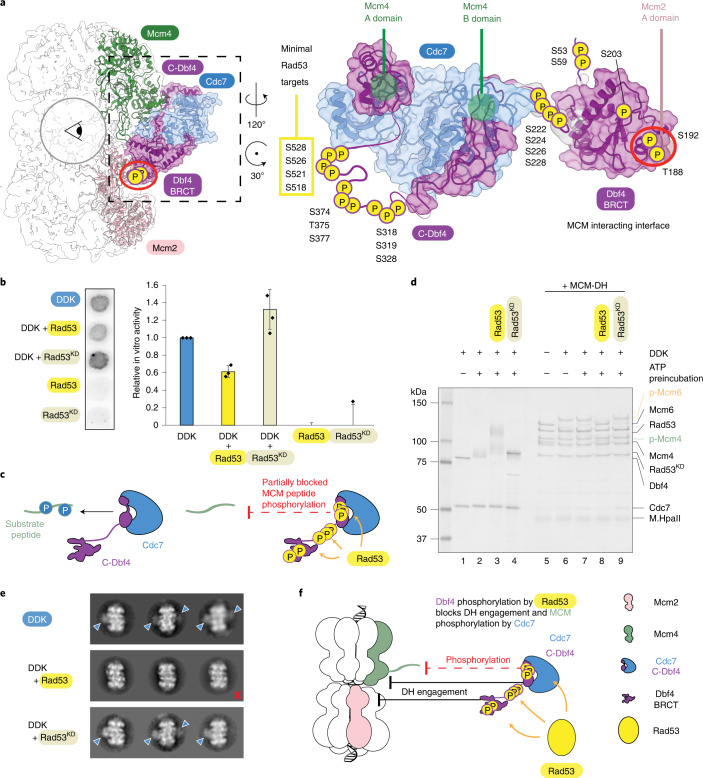
Fig. 6. DDK inhibition by Rad53.
a, Rad53 phosphotargets on Dbf4. S518–S528 phosphorylation blocks origin firing in cells. b, Autoradiograph and quantification of a kinase assay comparing peptide phosphorylation after treatment of DDK with Rad53 or an enzymatically inactive version (Rad53KD). The average of three biological replicas is plotted and error bars shows s.d. c, Model showing how phosphorylation by Rad53 interferes with the Cdc7 active site, independent of DH engagement. d, Phosphorylation of DNA-loaded MCM DHs is inhibited after Rad53-dependent phosphorylation of DDK, but not by the presence of Rad53KD. The effect was observed in three independent experiments. e, Table summarizing engagement of DDK with MCM DHs in the presence of Rad53 and Rad53KD when imaged by negative-stain EM. Representative 2D class averages of DHs and DDK-engaged DHs are shown from 6,033 (no Rad53), 7,018 (WT Rad53) and 5,519 (kinase-dead Rad53) picked DHs. Blue arrowheads indicate DDK bound to the DH and the red cross indicates DH without DDK. f, Model showing how Rad53-dependent phosphorylation of Dbf4 prevents DDK from docking onto the MCM and inhibits MCM phosphorylation. Uncropped images for b and d are available with the paper online.