Abstract
The present study describes the distribution and cellular morphology of catecholaminergic neurons in the diencephalon and midbrain of the bottlenose dolphin (Tursiops truncatus). Tyrosine hydroxylase immunohistochemistry was used to visualize these putatively dopaminergic neurons. The standard A1-A17, C1-C3, nomenclature is used for expediency; however, the neuroanatomical names of the various nuclei have also been given. Dolphins exhibit certain tyrosine hydroxylase immunoreactive (TH-ir) catecholaminergic neuronal groups in the midbrain (A8, A9, A10) and diencephalon (A11, A12, A14), however, no neuronal clusters clearly corresponding to the A13 and A15 groups could be identified. The subdivisions of these neuronal groups are in general agreement with those of other mammals, but there is a high degree of species specificity. First, three TH-ir neuronal groups not identified in other species were found: in the ventral lateral peri-aqueductal gray matter, posterior dorsal hypothalamus, and rostral mesencephalic raphe. Second, the normal components of the substantia nigra (A9 or pars compacta, A9 lateral or pars lateralis, A9 ventral or pars reticulata) were extremely cell sparse, but there was a substantial expansion of the A9 medial and A10 lateral subdivisions forming an impressive ‘ventral wing’ in the posterior substantia nigra. The findings of this and previous studies suggest a distinct evolutionary trend occurring in the neuromodulatory systems in mammals. The results are discussed in relation to motor control, thermoregulation, unihemispheric sleep, and dolphin cognition.
Keywords: Cetaceans, Dolphins, Tursiops, Mammals, Evolution, Dopamine
Introduction
Catecholaminergic neurons have been reported in the midbrain and diencephalon of all vertebrate species studied [Smeets and Reiner, 1994; Smeets and Gonzalez, 2000]. The presence of these neurons in various species has been identified with the use of the formaldehyde-induced fluorescence method, and in more recent years by tyrosine hydroxylase immunohistochemistry; however, studies of large-brained species, those with brains weighing more than 1 kg, have been limited to the human brain [Kitahama et al., 1994]. The present study reports the subdivisions of the catecholaminergic neuronal groups in the midbrain and diencephalon of the bottlenose dolphin, which at an average species brain weight of 1.5 kg represents the largest brain to date in which this system has been examined.
Evolutionary trends of the catecholaminergic systems, indeed of any of the neuromodulatory systems, are yet to be conclusively identified [Smeets and Reiner, 1994]; however, studying a range of vertebrate species, especially those that have undergone punctuated secondary enlargements such as the brains of cetaceans [Marino et al., 2000b], might reveal insights into the nature of evolutionary changes in these systems. In a previous paper examining the locus coeruleus complex (LC) of the bottlenose dolphin [Manger et al., 2003] it was proposed that changes in the complexity of the nuclear subdivisions of the LC were not linked to increases in brain size, rather, that these changes were linked to phylogenetic history with changes in nuclear complexity occurring at the establishment of a new mammalian order in a punctuated manner. This concept of evolutionary change [Gould, 2002] might provide more fruitful insights into the evolution of the catecholaminergic systems than the neo-Darwinian gradualistic analyses previously undertaken [Smeets and Reiner, 1994; Smeets and Gonzalez, 2000].
Many of the catecholaminergic neurons of the midbrain, specifically those of the substantia nigra and ventral tegmental area, have been demonstrated to be dopaminergic in nature [Dahlström and Fuxe, 1964; Fuxe et al., 1970; Ungerstedt, 1971; Lindvall and Björklund, 1974a, 1978; Björklund and Lindvall, 1984]. These dopaminergic neurons have been shown to be involved in a range of essential cognitive skills such as motor planning, working memory, cognitive flexibility, abstract representation, temporal analysis/sequencing, and generativity [reviewed in Previc, 1999]. They have also been shown to be essential in counteracting hyperthermia, and thus have been implicated in thermoregulatory mechanisms [also reviewed in Previc, 1999]. These corollaries of dopaminergic neuronal activity in the mammalian brain are of interest when examining the brains of cetaceans because: (1) Our cetacean example, the bottlenose dolphin, must maintain a mammalian core body temperature of around 36.9°C [Ridgway, 1972] while living its entire life in water where conductive heat loss is 90.8 times the rate of heat loss in air at the same ambient temperature [Downhower and Blumer, 1988]; (2) These animals have evolved an extreme form of unihemispheric sleep in which one hemisphere can be asleep while the other is awake [Mukhametov et al., 1977; Mukhametov, 1984; Ridgway, 2002]; and (3) Behavioral and psychophysical experiments have been interpreted to suggest that dolphins possess cognitive capabilities showing convergence with those exhibited by primates [Schusterman et al., 1986; Herman et al., 1999, 2001; McCowan et al., 2000; Marino, 2002]. On the other hand, there are no evident or noncontradictory examples of convergence in neural substrates when comparing dolphins with primates [Kruger, 1959, 1966; Kesarev, 1971; Kesarev et al., 1977; Morgane et al., 1982; Glezer et al., 1988].
The present paper provides the first detailed anatomical description of the catecholaminergic neuronal groups of the midbrain and diencephalon of the bottlenose dolphin. From this description we discuss the evolution of catecholaminergic systems and relate the details of these systems in the bottlenose dolphin to thermoregulatory mechanisms, unihemispheric sleep, and the proposed cognitive capacities of dolphins.
Materials and Methods
One specimen of an adult bottlenose dolphin (Tursiops truncatus) was used in the present study. This animal (NOR), a 151-kg adult female, was 245 cm in length and was collected from the Northern Gulf of Mexico as a young adult and maintained by the U.S. Navy Marine Mammal Program headquartered in San Diego, California for 13 years. This animal died of natural causes (1993; age approximately 23 years) due to the complications of a second pregnancy and parturition, despite a successful pregnancy three years earlier. The brain was removed 5 h postmortem, weighed (1,305 g; smaller than the overall species average of about 1,500 g) and partially sectioned along the interhemispheric cleft to expose the ventricles. The brain was immersion fixed in ten liters of Streck Tissue Fixative (Streck Laboratories Inc. Omaha, Nebraska). Five years following fixation (1998) the diencephalon and brainstem (midbrain, pons and medulla oblongata) were dissected away from the remainder of the brain and sectioned as described below.
Serial 50-μm sections of the diencephalon and brainstem (from the level of the enlarged posterior commissure through to the cervical spinal cord) were made in a coronal plane. Three consecutive sections from every ten were stained for Nissl substance with cresyl violet, fibers [Gallyas, 1979], or immunocytochemically stained for tyrosine hydroxylase (TH). For TH staining, the sections were rinsed 3 times in 0.1 M Trizma-buffered saline (TBS) followed by a 48-hour incubation at 4 ° C with a 1/500 dilution of primary rabbit antiserum to TH (Eugene Tech International Inc, Ridgefield Park, New Jersey). The dilutions were prepared with a solution of 1% normal goat serum (NGS) and 0.25% Triton X-100 in 0.1 M Tris-saline. This was followed by a 2.5-hour incubation with biotinylated goat anti-rabbit IgG (Vector Labs, Burlingame, California) diluted 1/200 with 1% NGS in Tris-saline. The tissue was then incubated for 2 h with the avidin-biotin complex diluted 1/100 with 1% NGS in Tris-saline (Vector). Between each incubation the sections were rinsed 3 times with 1% NGS in Tris-saline. The sections were then treated for 6 min with a 0.05% solution of 3,3′-diaminobenzidine and 0.01% hydrogen peroxide, rinsed in phosphate buffer, mounted to gel-coated slides, cleared in xylene and coverslipped with Depex mounting medium. The stained sections were examined under a low-power dissecting microscope, TH-immunoreactive (TH-ir) neuronal bodies marked using a camera lucida, and then matched to architectural boundaries determined from adjacent Nissl- and fiber-stained sections. This same specimen was used in a previous report detailing the appearance of the locus coeruleus complex in the dolphin [Manger et al., 2003].
Results
Orientation of the Sections
The outlines of the sections provided in the figures at first glance might seem unusual for coronal sections through the mammalian diencephalon and midbrain. This is due to the maintenance of the cephalic flexure in the adult cetaceans, which is lost in other mammals during ontogeny, thus rendering the hypothalamic portion of the diencephalon in a position ventral to the midbrain in the coronal plane. To prevent confusion and to aid in orientation, the plane of section in comparison to the dolphin whole brain, and the region of the brain sectioned and reported upon in this study are provided (fig. 1). This was not necessary in a previous study of the locus coeruleus complex in the rostral rhombencephalon [Manger et al., 2003], as the plane of section remained very close to that seen through this region in other mammals.
Fig. 1.
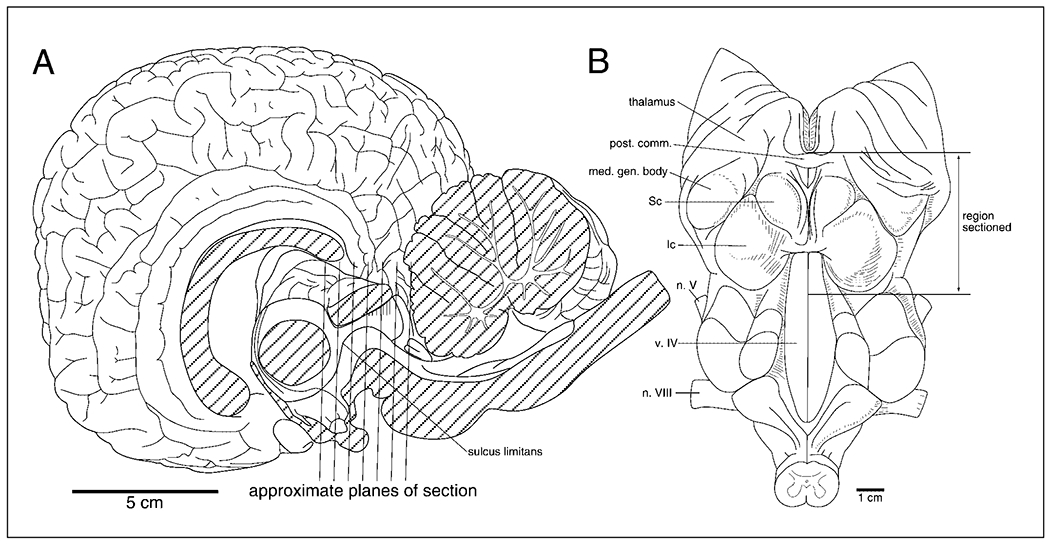
Drawings of the anatomy of the dolphin brain in mid-sagittal section (A) and the floor of the fourth ventricle (B). These figures are provided to allow orientation to the coronal sections of figure 2. As can be seen in A the hypothalamus is found ventral to the midbrain in the coronal plane due to maintenance of the cephalic flexure in the adult cetacean brain. These figures are redrawn from those of Breathnach [1960]. See list for abbreviations.
Catecholaminergic Cell Groups
TH immunohistochemistry revealed the existence of several clusters of catecholaminergic neurons within the diencephalon and midbrain of the dolphin. These include groups that we could identify as homologues in other mammalian species, and groups for which no homologue was clearly apparent. There were six groups with identifiable homologues in other mammals which include A14, A12 and A11 within the diencephalon, and A10 (4 subdivisions), A9 (4 subdivisions) and A8 within the midbrain. The most striking feature of these groups was the formation of a high density ventral wing of the A9 and A10 groups at the midbrain/pontine border making the division between the A9 and A10 groups indistinct [a feature noted in several other non-rodent mammals; Kitahama et al., 1994]. There were three groups for which we could not identify unambiguous homologues in other mammalian species and thus conclude that these are specific to the bottlenose dolphin although they are likely to be common to all cetaceans. However, in some cases there are reports of catecholaminergic cells in these regions, and these are mentioned. These groups include a ventral lateral periaqueductal gray matter cluster (VLpag), a posterior dorsal hypothalamic cluster (PDH), and a rostral mesencephalic raphe cluster (RMR) spanning the diencephalon and midbrain.
A14, Rostral periventricular cell group. This cluster of TH-ir neurons is found adjacent to the wall of the third ventricle, as in other species, and forms a cluster a few cell layers thick (fig. 2C–E). This cluster of neurons forms an antero-posterior column along the wall of the ventricle in the preoptic region, beginning just anterior to the optic chiasm, and ending at the posterior edge of the optic chiasm by commingling with neurons of the A12 subdivision (see below). The neurons of this cluster are very small, appear to be multipolar, but the dendritic branching is very sparse (fig. 3E, F).
Fig. 2.
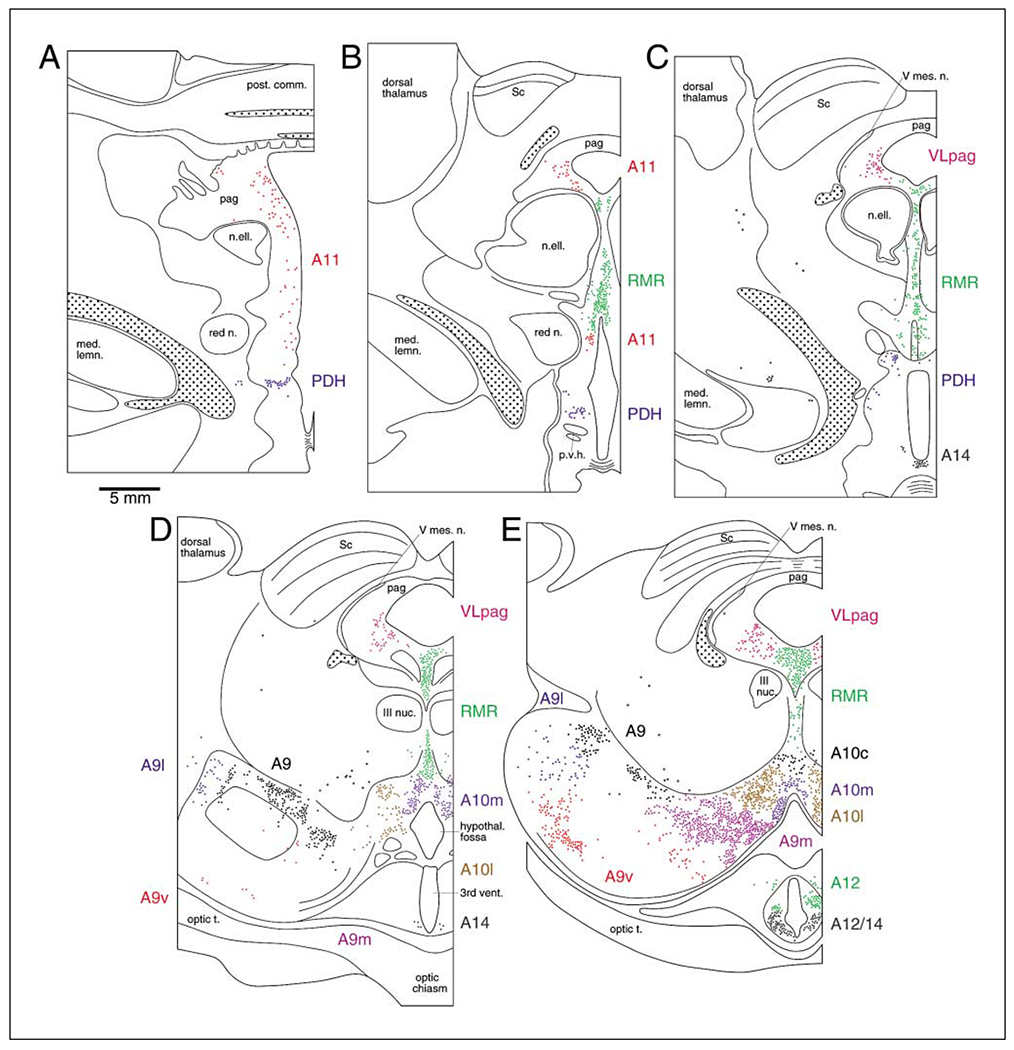
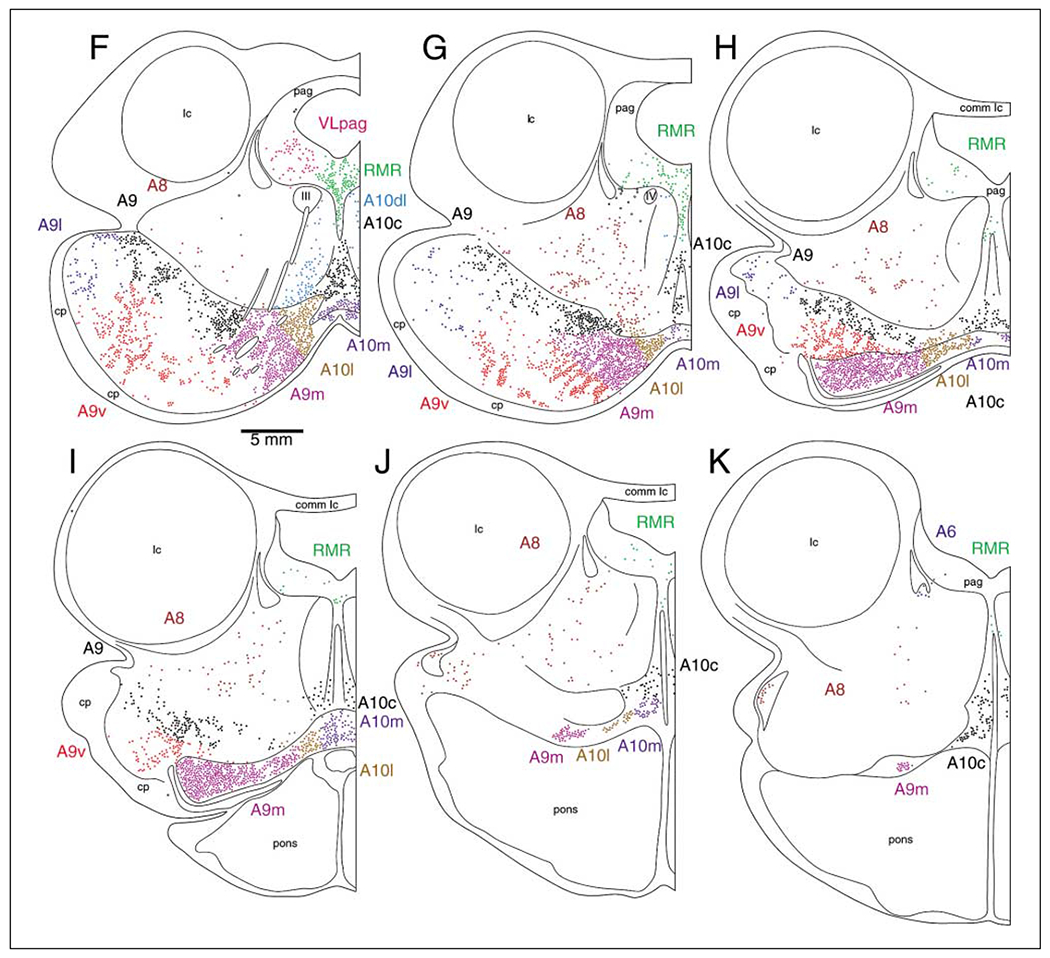
A series of coronal sections through the diencephalon and midbrain of the bottlenose dolphin showing the location of TH-immunopositive neurons found in the present study. A is the most rostral section, K the most caudal. Each subdivision of the catecholaminergic system has been drawn in a different color to allow ease of interpretation. The borders between cell groups, as defined by changes in color, were placed as close to the observed anatomical borders as possible. However, some of the borders between neuronal clusters were not as distinct as indicated here, thus the borders provided are meant more as a guide than as a stricture. Each colored dot equals one cell. The stippled regions indicate areas of high density TH-ir axonal staining and outline the medial forebrain bundle (A–C) and a bundle emanating from the locus coeruleus complex passing anteriorly to decussate across the posterior commissure (A–K). See list for abbreviations.
Fig. 3.
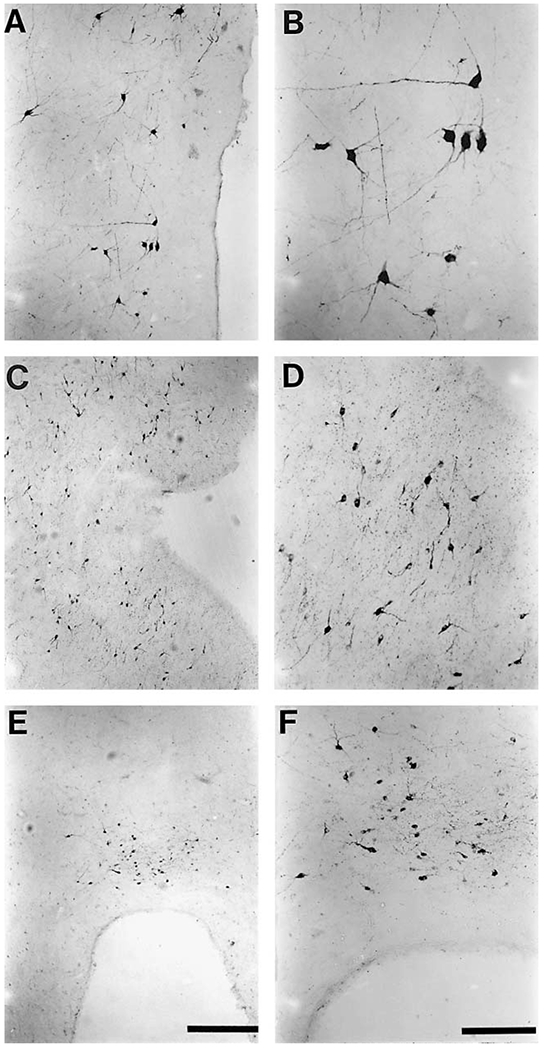
Photomicrographs of representative TH-ir neurons from the various subdivisions of the diencephalon and midbrain of the bottlenose dolphin. Both low power micrographs (A, C, E) which give an indication of density and high power micrographs (B, D, F) showing the cellular structure are provided for the A11 (A, B), A12 (C, D) and A14 (E, F) clusters that were observed. Note the large size of the A11 neuronal bodies (B) in comparison to those of the A12 (D) and A14 (F) neuronal bodies. Scale bar in E = 500 μm and applies to A, C and E; scale bar in F = 200 μm and applies to B, D and F.
A12, Tuberal cell group. The TH-ir neurons of this group formed a posterior continuation of the column of neurons of the A14 group, and are commingled with these neurons at the anterior limit of this group (fig. 2E). They are located just caudal to the optic chiasm, surrounding and adjacent to the walls of the third ventricle, lying in the floor of the hypothalamus as in the rat [Dahlström and Fuxe, 1964; Fuxe, 1964; Fuxe and Hökfelt, 1966]. These neurons have a limited antero-posterior range, i.e., less than 3 mm although within this range they exhibit a high density. The neurons of this group exhibit small bodies, and are bi- or multipolar in nature. The dendritic branching forms a sparse local plexus (fig. 3C, D).
A11, Caudal diencephalic group. This group formed a dorso-ventral column, almost 15 mm in height, of peri- and para-ventricular TH-ir neurons (fig. 2A–B). These are located along the wall of the third ventricle and are scattered and low in density. Lateral to the dorsal aspect of this column of neurons is the beginning of the large nucleus ellipticus, a specialization of the peri-aqueductal gray matter in cetaceans [Ridgway, 1990]. Lateral to the ventral aspect of this column is the red nucleus. Posteriorly, the column is split by the expansion of the nucleus ellipticus into dorsal and ventral portions (fig. 2B). The neurons of this group, both dorsally and ventrally have large soma and are multipolar. There is extensive branching of the dendrites and there is some orientation preference of the dendrites to the wall of the ventricle in the periventricular neurons (fig. 3A, B). The general appearance of these neurons is not dissimilar to that of the same group in rats [Dahlström and Fuxe, 1964]. There are some substantially smaller neurons commingled with the larger ones, but these appear to be part of the rostral mesencephalic raphe cluster (RMR, see below).
A10, Ventral tegmental area. The A10 clusters of TH-ir neurons were readily compartmentalized into four subdivisions: A10m (medial), A10l (lateral), A10d1 (dorsal lateral) and A10c (caudal). These components were found from the anterior-most level of the oculomotor nucleus and the mid-level of the optic chiasm, to the posterior border of the midbrain (6 mm posterior to the most anterior portion of the basilar pons; fig. 2D–K). The subdivisions of the A10 group of neurons were based on topology and neuronal morphology.
The A10 medial subdivision was formed by a cluster of neurons located immediately dorsal to the hypothalamic fossa anteriorly (within 2 mm of the floor of the midbrain), extending posteriorly close to the floor of the medial midbrain to the level of the A10/A9 ventral wing (see below). These neurons were located within 2 mm of the midline. The density was moderate but consistent throughout this cluster, and the caudal-most portion of this group was seen to form the medial-most portion of the A9/A10 ventral wing. The neurons were small in size, but multipolar in nature and exhibited a rich dendritic plexus (fig. 4A, E). They were weakly stained in comparison to other subdivisions of the A10 group.
Fig. 4.
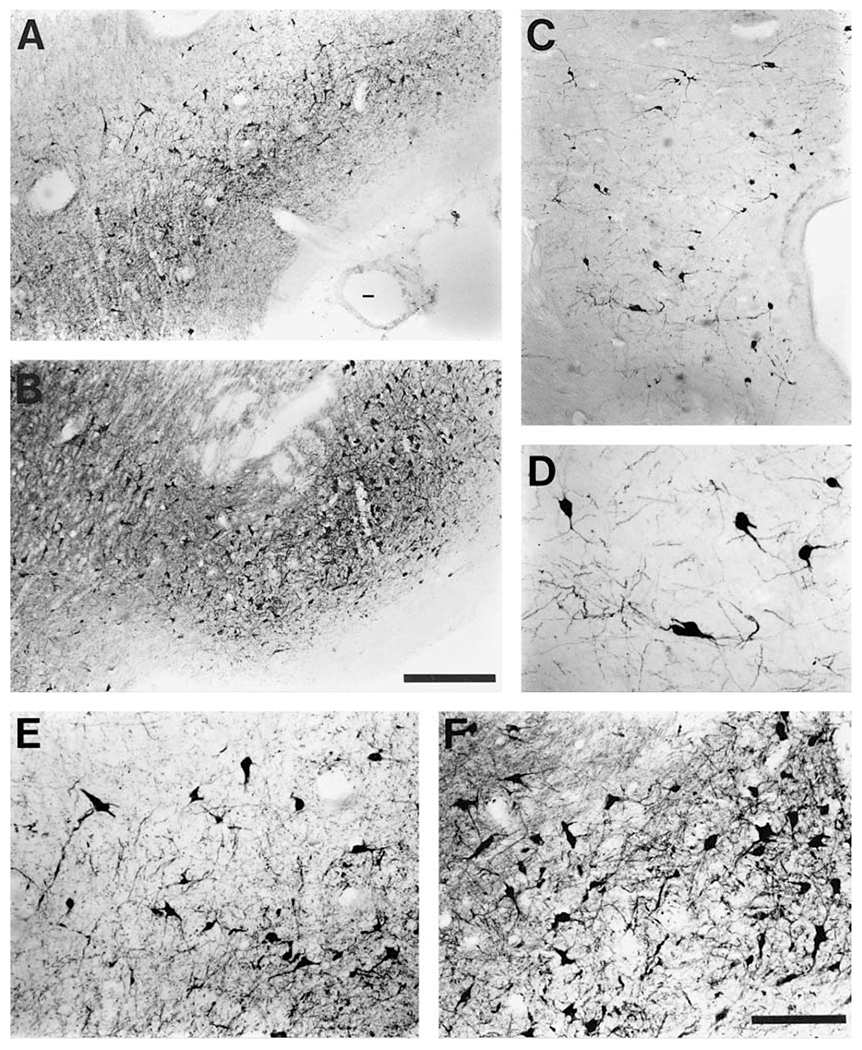
Photomicrographs of representative TH-ir neurons from two subdivisions of the ventral tegmental region (A10 group) of the bottlenose dolphin and the ventral lateral periaqueductal gray matter cluster (VLpag). Both low-power micrographs (A, B, C) which give an indication of density and high-power micrographs (D, E, F) showing the cellular structure are provided for the A10 medial (A, E), A10 lateral (B, F) and VLpag (C, D) clusters that were observed. Note the similarity in size of the A10 medial and lateral neuronal bodies, but the larger size of the VLpag neuronal bodies. The density of neurons is lower in VLpag than the subdivisions of the A10 region. Scale bar in B = 500 μm and applies to A, B and C; scale bar in F = 200 μm and applies to D, E and F.
The A10 lateral subdivision was found immediately lateral to the A10 medial cluster, dorsal to the most caudal parts of the paraventricular hypothalamic organ, and extended caudally in a loosely arranged fashion to form part of the medial portion of the A9/A10 ventral wing. This cluster showed a similar density of neurons to the A10 medial group, but was more expansive, extending from the floor of the midbrain to the ventral portion of the midbrain tegmentum, a distance of around 3 mm, and were located 2 to 5 mm from the midline. The oculomotor nerve was seen to pass just lateral to this cluster. The neurons of this group exhibited medium-sized soma, were multipolar with a rich dendritic plexus, and stained strongly for tyrosine hydroxylase (fig. 4B, F). The corresponding groups in rats have been termed nucleus para-brachialis pigmentosis and nucleus paranigralis [Fuxe et al., 1969].
The A10 dorsal lateral group was found lateral and dorsal to the groups described above and was located in the midbrain/pontine tegmentum (fig. 2F). These neurons were found in a para-raphe location, lateral to the raphe nuclei and bounded laterally by the oculomotor nerve. The number of neurons was few and scattered in a low density in this region. The neurons of this division exhibited smaller soma and moderate to weak dendritic branching.
The A10 caudal subdivision was comprised of neurons located in the ventral raphe dorsal to the mid-level of the A10 medial division at approximately the level of the oculomotor nucleus. The number of neurons was not great and the density was low. The neurons had medium-sized somas, exhibited a rich dendritic plexus, and stained moderately for tyrosine hydroxylase.
A9, Substantia nigra. The general configuration of the substantia nigra in the bottlenose dolphin bears many similarities to that of other mammalian species and can be compartmentalized into four subdivisions: A9 (pars compacta), A9 lateral (pars lateralis), A9 ventral (pars reticulata) and A9 medial (fig. 2D–K). Posteriorly, the A9 medial subdivision fuses with the A10l and A10m subdivisions to form an extraordinary ventral wing of the midbrain catecholaminergic system. However, the remainder of the substantia nigra of the dolphin is not as noticeably developed as in other mammals. The anterior limit of the A9 group is found at the level of the oculomotor nucleus and the optic chiasm. The posterior limit is found 6 mm posterior to the most anterior portion of the basilar pons, at the midbrain/pontine junction. Medially it is bounded by the A10 group, and laterally by the edge of the midbrain. In comparison to other mammals, the basis pedunculi of the cerebral peduncles in bottlenose dolphins are very small and it might appear that the ventral border of the substantia nigra is not formed by the basis pedunculi, as this is formed by the floor of the midbrain. However, a 1- to 1.5-mm strip of white matter, presumably the vestige of the basis pedunculi, lies between the ventral border of the substantia nigra and the floor of the midbrain. This strip, which we consider the basis pedunculi of the cerebral peduncle, forms the ventral border of the substantia nigra. At the most lateral and posterior portion this strip of basis pedunculi widens to form a small bulge on the lateral aspect of the midbrain immediately anterior to the most lateral aspect of the pons (fig. 2H–I). We could find no signs of neuromelanin in the substantia nigra.
The A9 pars compacta subdivision is found immediately ventral to the midbrain tegmentum, stretching as a poorly defined arc from 6–7 mm (marked by the passage of the oculomotor nerve) to 17–18 mm from the midline. This arc is approximately 2 mm in thickness. The number of TH-ir neurons in this subdivision is not numerous, and they exhibit a patchy distribution. The pars compacta is clearly not the dominant structure of the dolphin substantia nigra, unlike the situation seen in rodents, carnivores and primates [Kitahama et al., 1994; Tillet, 1994]. The TH-ir neurons of the A9 pars compacta subdivision have medium to large somas, are multipolar, and exhibit a moderate dendritic plexus (fig. 5A, B). The neuropil of this region is strongly immunoreactive for TH, especially in the anterior most portion, where the ascending projections of the midbrain coalesce to form the dopaminergic projection to the telencephalon.
Fig. 5.
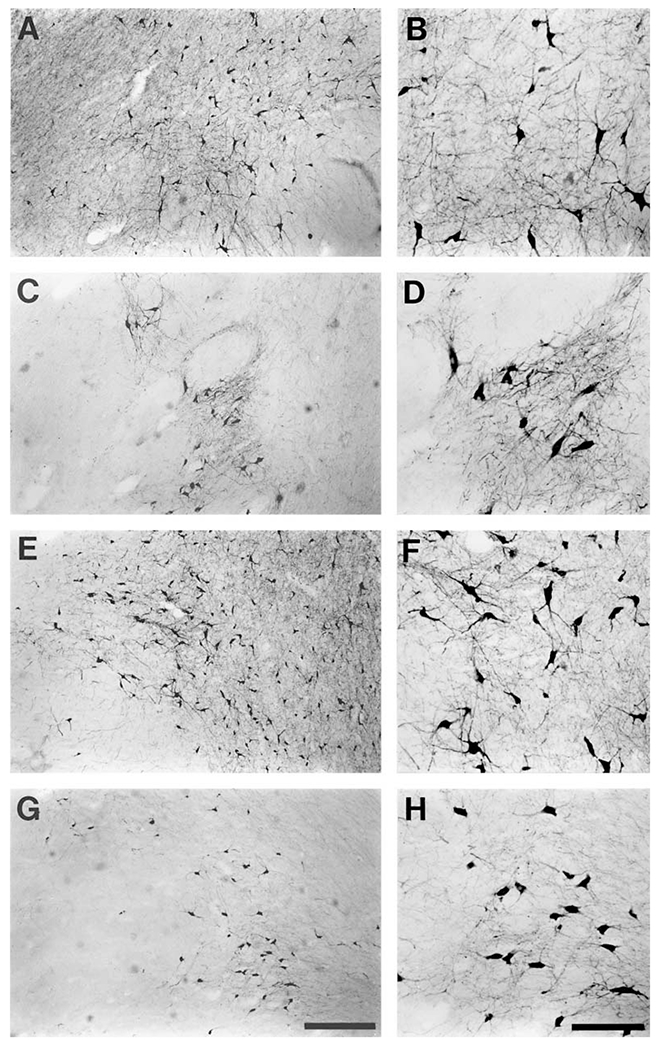
Photomicrographs of representative TH-ir neurons from the various subdivisions of the substantia nigra (A9 group) of the bottlenose dolphin. Both low-power micrographs (A, C, E, G) which give an indication of density and high-power micrographs (B, D, F, H) showing the cellular structure are provided for the A9 pars compacta (A, B), A9 ventral (C, D), A9 medial (E, F) and A9 lateral (G, H) clusters that were observed. Note the similarity in neuronal body size of all clusters, but the variations in density. In general it can be said that the normal subdivisions of the substantia nigra of the bottlenose dolphin are not well expressed. Scale bar in G = 500 μm and applies to A, C, E and G; scale bar in H = 200 μm and applies to B, D, F and H.
The A9 lateral subdivision is composed of a loosely arranged and widely spread collection of TH-ir neurons located in the pars lateralis region of the substantia nigra. It is found lateral to the pars compacta, lateral and dorsal to the pars reticulata, and is comprised of very few neurons. The neurons within this subdivision show a ‘typical’ A9 phenotype, as they are multipolar with medium-to-large soma with moderate dendritic arborization (fig. 5G, H). There are also some smaller neurons within this region showing moderate dendritic arbors.
The A9 ventral subdivision exhibits a greater degree of expression in comparison to the two above described subdivisions, and lies within the pars reticulata region of the substantia nigra. It is bordered dorsally by the pars compacta, laterally by the pars lateralis, medially by the A9m subdivision, and caudally by the A10l subdivision. As mentioned above, the basis pedunculi of the cerebral peduncles of the dolphin are very small, but they do form the ventral border of the A9 ventral division. Within this division, the neurons are arranged heterogeneously, forming tightly packed clusters oriented at an angle of around 45° to the midline (fig. 6C, D). These clusters, or bands, are separated by neuron-sparse regions that are presumably filled with fibers of passage. This heterogeneity of neurons clearly identifies the ‘reticular’ nature of this subdivision. The neurons within this region are strongly immunoreactive for tyrosine hydroxylase, are multipolar, with medium to large soma, and exhibit extensive and significantly branched dendritic arbors (figs. 5C, D; 6C, D).
Fig. 6.
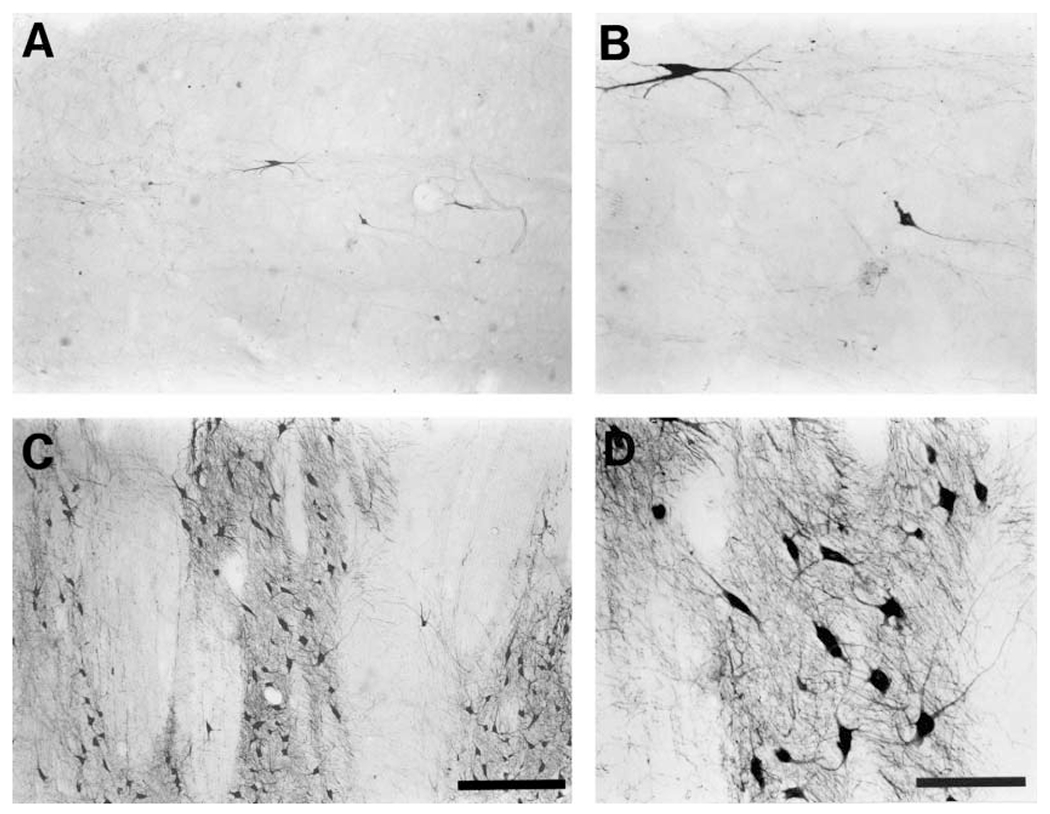
Photomicrographs of representative TH-ir neurons from the retrorubral region or A8 (A, B), and substantia nigra A9 ventral (pars reticulata) subdivision (C, D) of the bottlenose dolphin. Note the extremely low density and number of TH-ir neurons in the A8 region (A, B). The region of A9 ventral shown here exhibited striking striations (C, D) caused by the passage of the oculomotor nerve through this part of the substantia nigra (see also fig. 2F, G). Scale bar in C = 500 μm and applies to A, and C; scale bar in D = 200 μm and applies to B, and D.
The most striking feature of the substantia nigra, indeed the whole of the midbrain catecholaminergic system of the dolphin, is the extraordinary development of a ventral wing of strongly TH-ir neurons. This wing is composed of the A10m and A10l medially, but is mostly formed by an extensive lateral expansion of the A9 medial subdivision (fig. 2G℃J). The wing is located in a posterior position in the substantia nigra where it forms the most dominant structure. This ventral wing is bordered superiorly and anteriorly by the pars reticulata and the A10c, and ventrally by the floor of the midbrain anteriorly, and posteriorly by the pontine nuclei. It was not possible to detect a clear boundary between the A10 components of the wing medially and the A9 component laterally. The wing extends approximately 13 mm from the midline, and is 2–3 mm in height. Within this region the neurons are homogeneously distributed with a very high density, the majority exhibiting the ‘typical’ A9 appearance (figs. 5E, F; 7).
Fig. 7.
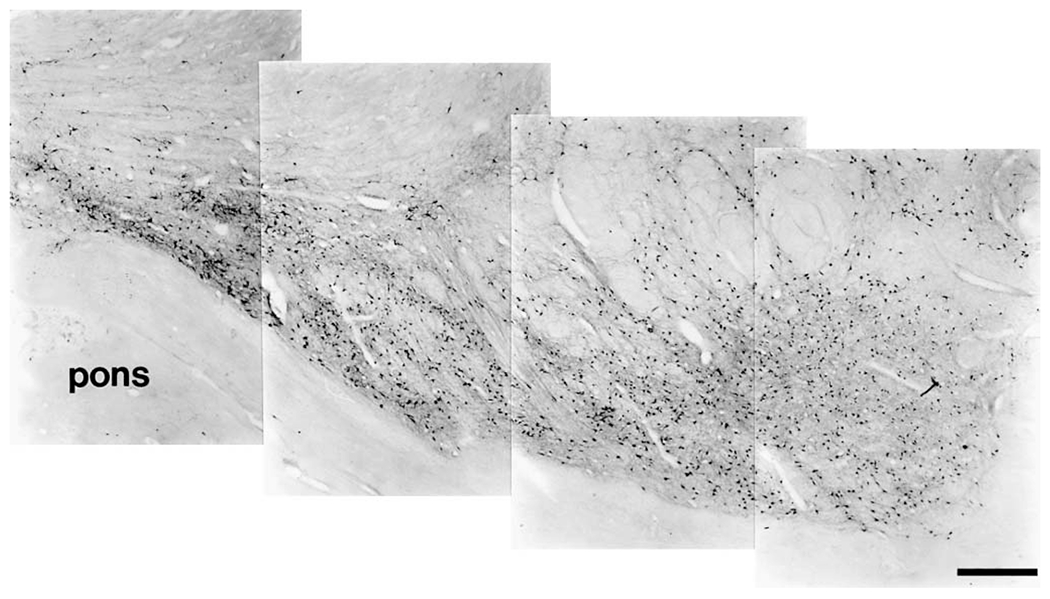
Photomontage of the TH-ir neurons that make up the ventral wing of the midbrain catecholaminergic system of the bottlenose dolphin. Medial is to the left and dorsal is up. Note the relatively higher density of TH-ir neurons in this region as compared with the remainder of the substantia nigra (see figs. 5 and 6). Immediately below this ventral wing is the cerebral peduncle (cp) and the pons. Scale bar = 1 mm.
A8, Retrorubral. A distinct A8 cluster of neurons was found in the tegmentum of the midbrain, located dorsally to the substantia nigra (fig. 2F–K). The neurons comprising this cluster were found throughout the midbrain tegmentum from the level of the trochlear nucleus to the midbrain/pontine junction, lying laterally to the oculomotor nerve. The anterior border of this region was marked by a lack of TH-ir neurons, whereas the posterior border was marked by a substantial decrease in the number of TH-ir neurons, prior to the increase in number of TH-ir neurons marking the beginning of the subcoeruleus [Manger et al., 2003]. Although spread across the entire midbrain tegmentum, these neurons were few in number and thus exhibited a very low density. The neurons of this subdivision had small-to-medium size soma and were multipolar. The smaller neurons had weaker dendritic branching, but the dendritic arborization of the larger neurons could be quite impressive (fig. 6A, B).
Ventral lateral periaqueductal gray cluster (VLpag). As mentioned above, we identified three clusters in the dolphin for which homologies with other species could not be readily made. The first cluster was comprised of a loosely arranged group of TH-ir neurons, with a moderate to low density, spanning the ventral lateral periaqueductal gray matter from the ependymal wall of the aqueduct to the edge of the gray matter (fig. 2C–F). This cluster was located in the region immediately dorsal to the oculomotor nucleus. The fifth mesencephalic nucleus was found immediately dorsal and lateral to VLpag. This group appears to be a continuation of the dorsal most portion of the A11 cluster of neurons, but is distinguished from these by their neuronal morphology. The neurons of this subdivision were mostly medium sized and multipolar, and there was no apparent orientation in the direction of the moderately branched dendritic arbors (fig. 4C, D).
Posterior dorsal hypothalamic cluster (PDH). The second cluster was found in the posterior dorsal hypothalamus (fig. 2A–C). This cluster was located in the gray matter adjacent to the aqueduct, in a position ventro-medial to the red nucleus, ventral to A11, and immediately dorsal to the paraventricular hypothalamic nucleus. This cluster had few TH-ir neurons, and was mostly seen as a loosely clumped, medio-laterally oriented band, approximately 2.5 mm in width and 1 mm in height. The cellular morphology clearly distinguished this cluster from others staining for tyrosine hydroxylase in the vicinity. The soma of the neurons were mostly small, but some medium-sized somas were also evident. The neurons were multipolar and there was moderate dendritic branching (fig. 8A, C).
Fig. 8.
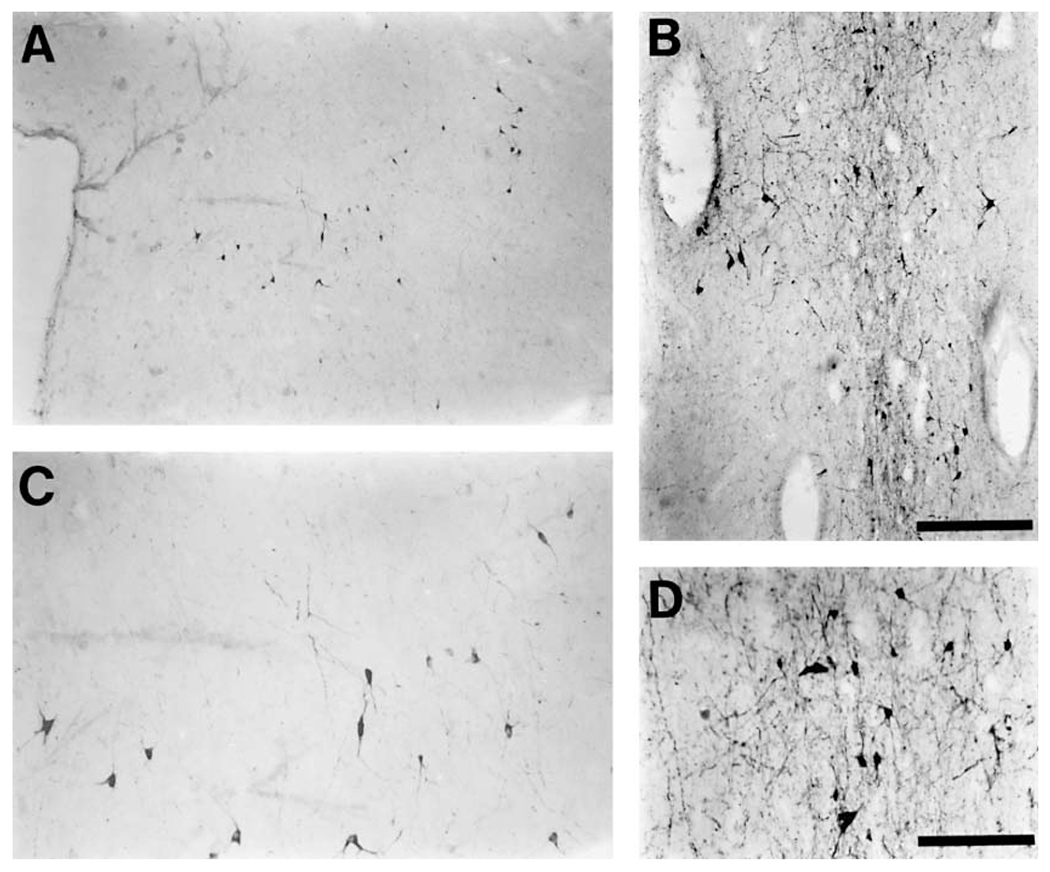
Photomicrographs of representative TH-ir neurons from two of the previously unidentified catecholaminergic groups of the bottlenose dolphin, the posterior dorsal hypothalamic cluster (A – low power and C – high power), and the rostral mesencephalic raphe cluster (B – low power and D – high power). Note the small size of these neurons in comparison to those of the substantia nigra and ventral tegmental area neurons (magnifications in figs. 4, 5 and 6 are the same as in this plate), giving them the appearance of dopaminergic interneurons, a supposition that is supported by the small size of the dendritic arborization. Scale bar in B = 500 μm and applies to A, and B; scale bar in D = 200 μm and applies to C, and D.
Rostral mesencephalic raphe cluster (RMR). The third group of dolphin TH-ir neurons with no clear homologue in other mammals was found within the rostral most portions of the raphe. This cluster of numerous neurons formed a loosely arranged column within 1.5 mm of either side of the midline from the mid-level of the nucleus ellipticus and red nucleus to the midbrain/pontine junction (fig. 2B–K). The column extended from close to the ependymal surface of the aqueduct to the level of the third ventricle anteriorly, and to the level of the A10c cluster posteriorly. In the middle portions of this cluster, the number of neurons within the periaqueductal gray matter increased significantly. However, towards the posterior portions these neurons decreased in number. The morphology of these neurons distinguished them from other TH-ir neurons in the vicinity. The somata of these neurons were small to medium in size and were multipolar. The dendritic aborization was not extensive, but the dendrites did show some orientation along the dorso-ventral plane within the raphe (fig. 8B, D).
Discussion
The Homologized Groups within the Cetaceans
The principal architectural and distributional pattern of the TH-immunoreactive (TH-ir) neuronal cell groups of the dolphin diencephalon and midbrain was similar to that of other mammals, where they have been shown to represent dopaminergic neurons [Dahlström and Fuxe, 1964; Fuxe et al., 1970; Lindvall and Björklund, 1974b; Björklund and Lindvall, 1984]. In the diencephalon the A11 group could be identified. This group is known to project to the spinal cord [Björklund and Skagerberg, 1979; Hökfelt et al., 1979; Lindvall et al., 1983] and the A12 cell group that projects to the median eminence [Fuxe, 1964; Fuxe and Hökfelt, 1966] and the pituitary gland [Björklund et al., 1974]. The small clusters of TH-ir neurons in the hypothalamic periventricular region of the dolphin brain appear to be homologous to the A14 dopaminergic neurons described in the rat [Fuxe, 1964; Björklund et al., 1973; Hökfelt et al., 1976; Björklund and Lindvall, 1984]. No evidence of the laterally located A15 cell group was found in this region of the dolphin brain [Hökfelt et al., 1984]. The existence of an A13 group in the zona incerta [Fuxe et al., 1969; Björklund et al., 1975] of the dolphin remains to be determined, although the posterior dorsal hypothalamic neuronal cluster (PDH) observed in the dolphin hypothalamus might in part correspond to the A13 group. Thus, several similar hypothalamic dopaminergic neuronal groups, probably with functions similar to those in other mammals, appear to exist in the dolphin and these might play a role in neuroendocrine functions [Fuxe et al., 1970; Björklund et al., 1974]. Some of these dopaminergic neuronal groups could have a role in thermoregulation, as it seems likely that D2 receptors in the lateral hypothalamus in mammals mediate hypothalamic responses [Fuxe and Sjöqvist, 1972; Cox and Lee, 1979; Faunt and Crocker, 1987; Salmi et al., 1993; Parada et al., 1995].
The A9 group in the substantia nigra of the dolphin is comparably developed to that seen in other mammals [Dahlström and Fuxe, 1964; Andén et al., 1964; Fuxe et al., 1970; Battista et al., 1972; Lindvall and Björklund, 1974b; Björklund and Lindvall, 1984] forming the large ascending dopaminergic axonal bundles that traverse the ventral thalamus. There is, however, a major difference in the reduced differentiation and expression of the common nigral groups, with the pars compacta, pars lateralis and pars reticulata region exhibiting few TH-ir neurons. However, there is an impressive accumulation of TH-ir neurons in the uniquely formed ventral wing, especially in the caudal and medial part of the substantia nigra in continuity with the lateral A10 group. The functional consequences of this altered nigral anatomy in the dolphins is unclear, but we can speculate that it could involve the need for a dopaminergic modulation of movement of the entire body as opposed to specialized limb movement. Alternatively, the ventral wing might be involved in the modulation (nigral portion) and motivational (ventral tegmental portion) aspects of the complex movement of air in the cetacean blowhole that corresponds with the production of sound. Overall there appears to be a significant dopaminergic projection from the substantia nigra to the striatum in the dolphin brain indicating that the nigrostriatal system, as observed in other mammals, could play a similar movement modulation role in the dolphin.
The A10 dopaminergic neuronal group, the ventral tegmental area, exhibited subdivisions similar to the A10 groups of other mammals. The only subdivision that is consistently identified in other mammals that was not observed in the dolphin was the A10 dorsal caudal cluster. The A10 group [Dahlström and Fuxe, 1964] gives rise to the mesolimbic dopaminergic system projecting to subcortical structures, especially to the nucleus accumbens and olfactory tubercle, and also to regions of the cerebral cortex [Dahlström and Fuxe, 1964; Andén et al., 1966; Fuxe et al., 1970; Ungerstedt, 1971; Lindvall and Björklund, 1974a, 1978; Björklund and Lindvall, 1984]. This system is inter alia involved in motivational aspects of motor behavior [see Ferré, 1997] and mediates the rewarding actions of psychostimulant drugs. The present analysis indicates that the mesolimbic dopaminergic system of the dolphin, at least at level of the nuclear parcellation of the dopaminergic neurons in the ventral tegmental region, is comparable to that of other mammals, although the limbic ‘lobe’ as a whole is reduced in cetaceans [Kruger, 1959, 1966; Morgane et al., 1982].
The dopaminergic neuronal group A8, the retrorubral cluster of the dolphin brain, has a similar appearance and location as that described in other mammals [Dahlström and Fuxe, 1964] and is thus much smaller than the A10 group. Its functional role still remains unknown but in other mammals it projects to both limbic regions and the antero-medial part of the corpus striatum [Lindvall and Björklund, 1978; Björklund and Lindvall, 1984].
The Three Groups That Appear to Be Unique in Dolphins
The TH-ir neurons observed in the ventral lateral periaqueductal gray matter (VLpag) observed in the dolphin appear similar to the caudal periventricular dopaminergic neurons described in the rat [Hökfelt et al., 1976; Lindvall and Björklund, 1974a, 1978; Björklund and Lindvall, 1984] and do not form part of the A10 group. The VLpag appears to be a dopaminergic system of its own that might be called ‘A11 caudal’, although it is distinctly different in its morphology and projections (in other mammalian species) from the A11 group that is located rostral to this cluster. Although seen in the rat, this cluster of TH-ir neurons was not assigned a number due to the small number of neurons located in this region and the variability in their appearance. Thus, this group, the VLpag, represents a unique feature of the dolphin midbrain. Its possible function is unknown, but it appears to be part of a periventricular system in other mammals [see Björklund and Lindvall, 1984]. The posterior dorsal hypothalamic cluster could in part correspond to the A13 group in other mammals and might thus project into other hypothalamic areas [Björklund et al., 1975].
The large number of TH-ir neurons in the rostral mesencephalic raphe (RMR) are not part of the VLpag dopaminergic system but represent a group of their own. This rostral mesencephalic raphe group was not assigned a number in the studies of other mammals, where, although present, is less developed [Hökfelt et al., 1984]. It is therefore called the rostral mesencephalic raphe (RMR) cluster here. The significant expression of this group represents a second specialized feature of the dolphin midbrain. It appears to end caudally at the level where the A10 caudal group begins to extend dorsally toward the dorsal raphe nucleus.
Evolutionary Trends in the Catecholaminergic System
From the observations made in the present and previous studies [Kitahama et al., 1994; Manger et al., 2002, 2003] an increasing amount of evidence is becoming available that indicates a specific evolutionary trend exhibited by the catecholaminergic systems of mammals. This trend was first discussed in a previous study [Manger et al., 2003] and is suggested as an evolutionary constraint. Thus, we propose that mammals within the same phylogenetic order will display the same complement of nuclear subdivisions of the catecholaminergic systems irrespective of total brain size, adaptive external or internal phenotype, or lifestyle. Of course it is clear that certain nuclear subdivisions of the catecholaminergic systems will be found in all mammalian species [Smeets and Gonzalez, 2000], but it is the complement of these divisions that exhibits the evolutionary trend. Supportive evidence for this proposal comes from studies that examined species of the same order but of differing brain size. In a previous study we examined the catecholaminergic system of monotremes [Manger et al., 2002]. In that study we concluded that a total of 22 subdivisions of the catecholaminergic system were present in both the platypus and echidna. Importantly, the complement, i.e. the actual identity of these subdivisions, were the same in both monotreme species despite the fact that the echidna has a brain that weighs approximately three times that of the platypus. Moreover: (1) there were nuclear subdivisions that are consistently reported for eutherian mammals that were not found in the monotremes, such as those found in the habenular, basal forebrain, and cerebral cortex; (2) there were nuclear groupings that are variously reported for eutherian mammals that were also not present in the monotremes, such as those found within the dorsal accessory inferior olive (A3), the roof of the fourth ventricle (A4), and medio-dorsal to the medial longitudinal fasciculus (C3); and (3) there were nuclear groupings found in the monotremes that are reported only inconsistently in eutherian mammals, such as those found in the rostral dorsomedial region of the area postrema (C2) and the spinal cord.
In the present and a previous study [Manger et al., 2003] we report on a total of 19 nuclear subdivisions of the catecholaminergic system of the dolphin, but as the whole brain was not examined there are certain to be more. Of these 19 subdivisions, clear homologues in other eutherian mammals could be found for 16, although three subdivisions appear to be unique to the bottlenose dolphin: the ventral lateral periaqueductal gray, posterior dorsal hypothalamus, and the rostral mesencephalic raphe. Thus, the dolphin conforms mostly to the general organization of the eutherian mammalian catecholaminergic system, but there are three unique nuclei that distinguish the total complement of catecholaminergic nuclear subdivisions from that of all other mammals. There is also the unique expansion of the ventral wing of the substantia nigra and ventral tegmental area that is unique to the dolphin. Moreover, there is the absence of the A15, A13, and A10 dorsal caudal divisions. Thus, it can be stated that the complement of catecholaminergic nuclear subdivisions in the brain of the dolphin is unique among mammals studied to date.
A previous review of the mammalian catecholaminergic system by Kitahama et al. [1994] also reported discrepancies and similarities in the complement of nuclear subdivisions of various mammalian species. For example, Kitahama et al. point out that an A4 subdivision is found in rat, human and monkey, but is not found in sheep, nor was it seen in the bottlenose dolphin [Manger et al., 2003]. Moreover, Kitahama et al. indicate that the C3 group is found in mouse, rat and hamster, but not other mammalian species, making it a rodent-specific nucleus. Kitahama et al. also mention that there appear to be no nuclear subdivisions unique to the human that are not found in the monkey, despite an almost 15 times difference in brain size, with a similar conclusion drawn in a comparison of the dog and cat with a 3 times difference in brain size. The number of subdivisions found in the present study of the bottlenose dolphin does not appear to indicate that the midbrain catecholaminergic system is appreciably more complexly organized than that of the mouse, even though there is a 1,500 times difference in brain size.
Thus, we are left with the following suggestions: (1) brain size does not correlate with increased complexity in the organization of the nuclear subdivisions of the catecholaminergic systems within or between mammalian orders; (2) the complement of nuclear subdivisions differs among mammalian orders, but is consistent within a mammalian order; and (3) mammalian orders might exhibit unique nuclear subdivisions of the catecholaminergic system, but these are likely to be found in all species of that order. From these observations we can propose that changes in the nuclear complexity of the catecholaminergic system will occur only during the time of non-lethal genetic mutation that leads to the establishment of a new mammalian order, and that all species of that order will have the same complement of catecholaminergic subdivisions irrespective of the subsequently evolved size of the brain, resulting phenotype, or lifestyle. These ideas are yet to be tested in the other 80 or so members of the order Cetacea and members of other mammalian orders, but the preliminary evidence outlined above is supportive of these suggestions.
The Basis Pedunculi of the Cerebral Peduncles
An interesting observation made during the present study was the small size of the basis pedunculi of the cerebral peduncles of the dolphin. In other mammals, and presumably also in the dolphin, the basis pedunculi is comprised of the descending cortico-pontine and corticospinal tracts. Thus, it is apparent that the descending cortical motor tracts of the cetaceans are quite small. This observation of the small basis pedunculi is confirmed by earlier reports indicating small descending motor pathways [Hepburn and Waterston, 1904; Gierlich, 1916], although it is known that these pathways follow the typical mammalian pattern [Breathnach, 1960]. This decreased size of descending motor pathways appears to correlate with the lack of hindlimbs and greatly reduced forelimbs. However, the significant size of the pontine nucleus and the cerebellum [Marino et al., 2000a] are also of interest. It might be hypothesized that on the basis of small descending cortical motor pathways, a significantly sized pontine nucleus, and a relatively enlarged cerebellum, that programmed motor patterns control many cetacean movements such as rapid and precisely timed echo-location click production and head scanning for sonar echo reception [Ridgway and Au, 1999; Ridgway, 2000].
Dopamine and Thermoregulation
It is well established that dopamine is involved in thermoregulation. Specifically, dopamine is recognized as a major neurotransmitter involved in counteracting hyperthermia [Lee et al., 1985]. The observations made in the present study are of interest in this regard, as the dolphin lives in an aquatic environment where conductive heat loss is 90.8 times the rate it is in air at the same ambient temperature [Downhower and Blumer, 1988]. Thus, dolphins under normal conditions are not likely to suffer from heat stress, rather, they might have more difficulty generating heat than losing it. Hypothalamic dopaminergic mechanisms, as well as projections from the substantia nigra to the hypothalamus, have been shown to initiate heat loss mechanisms that can reduce core body temperature by up to 3°C [Lee et al., 1985]. Both the hypothalamic and common nigral dopaminergic nuclei in the dolphin are poorly differentiated and low in cellular numbers (from a qualitative judgment). Given the aquatic environment of the dolphin, with the low risk of hyperthermia and the relationship of dopamine to hyperthermic mechanisms, it appears not altogether too surprising that the differentiation of the hypothalamic and common nigral dopaminergic system in the dolphin is not comparable to that of terrestrial mammals.
Unihemispheric Slow-Wave Sleep and Dopaminergic Systems in Dolphins
One of the interesting and as yet not fully understood aspects of dolphin physiology and behavior is unihemispheric slow-wave sleep (USWS) phenomenology. Although the phenomenology of USWS and the small amount of REM sleep have been elucidated in the dolphin and several other cetacean species [Serafetinides et al., 1970; Goley, 1999; Lyamin et al., 2001], the neural circuitry that underlies this phenomen is not well understood. In a previous study [Manger et al., 2003] we described what appears to be a ‘normal’ locus coeruleus in the dolphin and related this appearance to the maintenance of muscle tone in the sleeping dolphin. This suggestion is supported by the ability of the dolphin to swim constantly during sleep periods, and therefore the need for continuous muscle tone maintenance.
Recent studies have shown that there are dopaminergic neurons that are sleep-state dependent which are located in the ventral periaqueductal gray matter [Lu et al., 2002]. Interestingly, in the present study we found a significant population of TH-ir neurons, potentially dopaminergic, in the ventral lateral periaqueductal gray that we term VLpag. Second, studies have also shown that the descending axons of neurons of the A11 subgroup are involved in abnormal sleep movements [Mignot et al., 2002]. The ability of dolphins to swim throughout all phases of sleep is clearly unique in comparison to other mammals. As yet we have not found any neural substrate that can be considered directly responsible for USWS, but the combined observations of the present paper, those on dolphin locus coeruleus [Manger et al., 2003], those in bi-hemispheric sleeping mammals [Mignot et al., 2002], and further study of the immunohistochemically identifiable neuronal populations involved in sleep regulation in the dolphins and other mammals, might disclose the circuitry underlying this most interesting form of sleep phenomenology.
Dolphin Playful/Hedonistic Behaviors and Cognition
Our findings suggest that the mesolimbic dopaminergic reward pathways might have a major role in the play behaviors of the dolphin [McCowan et al., 2000; Reynolds et al., 2001]. It seems reasonable to think that these ‘playful’ behaviors could reflect a sensory feedback activation of the A10 mesolimbic dopaminergic system, giving rise to reward signals in the nucleus accumbens that maintain these behaviors in dolphins. This speculation can be developed further as cetaceans appear to lack, or have a very small, prefrontal cortical region [Kojima, 1951; Lende and Akdikmen, 1968; Lende and Welker, 1972; Kesarev et al., 1977; Ladygina et al., 1978], which is confirmed by the small size of the hippocampal formation [Morgane et al., 1982]. The lack, or reduction, of prefrontal cortex in the dolphin could indicate that the rewarding stimuli produced by activity of the ventral tegmental dopaminergic neurons might not be under neocortical suppression as seen in other mammals, thus leading to consistent seeking of the activation of the dopaminergic reward system by the dolphin and the appearance of ‘hedonistic’ or ‘playful’ behavior [Schusterman et al., 1986; McCowan et al., 2000; Reynolds et al., 2001].
Previc [1999] has presented the hypothesis that an expansion of the nigro-striatal dopaminergic system and epigenetic factors leading to increased levels of dopamine during hominid evolution lies at the basis of current human intellectual abilities [but see Hardman et al., 2002, for a quantitative study of dopaminergic cell numbers in primates]. Dopamine is involved in such cognitive abilities as motor planning, working memory, cognitive flexibility, abstract representation, temporal analysis/sequencing, and generativity [Previc, 1999]. The relationship of dopamine to intelligence is of interest to the results of the present study, as certain observations [e.g., Marino, 1998; Herman et al., 1999, 2001; Reiss and Marino, 2001], have lead to the conclusion that the cognitive capacities of cetaceans and primates have converged [Herman et al., 2001; Marino, 2002]. The present study reveals a poorly differentiated nigral dopaminergic system in the bottlenose dolphin. Indeed, the major component of the substantia nigra in most mammals, the pars compacta, is very cell sparse in the dolphin. Thus: (1) We might conclude that given the behavioral observations on cetaceans the hypothesis of Previc is incorrect and that the dopaminergic system has no relevance to intellectual abilities of cetaceans or any other mammal; or (2) If we accept the hypothesis of Previc, that an expansion of the dopaminergic system is central to increased intellectual capacity, then as this system is poorly differentiated in the dolphin, the dolphin must not have the intellect suggested by the behavioral studies; or (3) We accept that both the hypothesis of Previc and the hypothesis that dolphins do have a relatively high intellect are true. We must then consider that the evolution of the intellect in humans and dolphins is convergent and that the evolution of brain structures other than the dopaminergic system led to increased cognitive capacities in dolphins.
Acknowledgments
This work was supported by the Medical Research Service of the Veterans Administration, USPHS grant NS32819, NIH grant NS42947 and NSF grant 0234687 and the U.S. Navy Marine Mammal Program. We thank Drs. William Van Bonn and Raymond Tarpley for help in dissection and brain removal, and Cheryl Short for attending the fixing brain. The authors would also like to thank Heidi Fahringer and Brenda Chestnut for expert technical assistance in the preparation of the histological sections used in this study.
Abbreviations
- A8
retrorubral
- A9
substantia nigra pars compacta
- A9l
lateral, substantia nigra pars lateralis
- A9m
medial, substantia nigra
- A9v
ventral, substantia nigra pars reticulata
- A10
ventral tegmental area
- A10c
caudal ventral tegmental area
- A10dl
dorsal lateral ventral tegmental area
- A10l
lateral ventral tegmental area
- A10m
medial ventral tegmental area
- A11
caudal diencephalic group
- A12
tuberal cell group
- A13
zona incerta
- A12/A14
region of commingled A12 and A14 neurons
- A14
rostral periventricular cell group
- 3rd vent
third ventricle
- comm Ic
commissure of the inferior colliculus
- cp
cerebral peduncle
- hypothal. fossa
hypothalamic fossa
- Ic
inferior colliculus
- III nuc. or III
third cranial nerve nucleus, oculomotor nucleus
- IV
fourth cranial nerve nucleus, trochlear nucleus
- med. gen. body
medial geniculate body
- med. lemn.
medial lemniscus
- n.ell.
nucleus ellipticus
- NGS
normal goat serum
- n. V
root of fifth cranial nerve, trigeminal nerve
- n. VIII
root of eighth cranial nerve, vestibulocochlear nerve
- optic n.
optic nerve
- pag
periaqueductal gray matter
- PDH
posterior dorsal hypothalamic cluster
- post. comm.
posterior commissure
- p.v.h
paraventricular hypothalamic nucleus
- red n.
red nucleus, nucleus ruber
- RMR
rostral mesencephalic raphe cluster
- Sc
superior colliculus
- TBS
Trizma-buffered saline
- TH
tyrosine hydroxylase
- V mes. n.
fifth mesencephalic nucleus
- v. IV
floor of fourth ventricle
- VLpag
ventral lateral periaqueductal gray cluster
References
- Andén N-E, Carlsson A, Dahlström A, Fuxe K (1964) Demonstration and mapping out of nigro-neostriatal dopamine neurons. Life Sci 3: 523–530. [DOI] [PubMed] [Google Scholar]
- Andén N-E, Dahlström A, Fuxe K, Larsson K, Olson L, Ungerstedt U (1966) Ascending monoamine neurons to the telencephalon and diencephalon. Acta Physiol Scand 67:313–326. [Google Scholar]
- Battista A, Fuxe K, Goldstein M, Ogawa M (1972) Mapping of central monoamine neurons in the monkey. Experientia 28:688–690. [DOI] [PubMed] [Google Scholar]
- Björklund A, Lindvall O (1984) Dopamine-containing systems in the CNS. In: Handbook of Chemical Neuroanatomy, Vol. 2: Classical Transmitters in the CNS, Part 1. (Björklund A, Hökfelt T, eds), pp 55–122. Amsterdam: Elsevier. [Google Scholar]
- Björklund A, Skagerberg G (1979) Evidence for a major spinal cord projection from the diencephalic A11 dopamine cell group in the rat using transmitter-specific fluorescent retrograde tracing. Brain Res 177:170–175. [DOI] [PubMed] [Google Scholar]
- Björklund A, Falck B, Nobin A, Stenevi U (1974) Organization of the dopamine and noradrenaline innervations of the median eminence-pituitary region in the rat. In: Neurosecretion – The Final Neuroendocrine Pathway. VIth International Symposium on Neurosecretion, London, 1974. Berlin/Heidelberg/New York: Springer-Verlag. [Google Scholar]
- Björklund A, Lindvall O, Nobin A (1975) Evidence of an incerto-hypothalamic dopamine neurone system in the rat. Brain Res 89:29–42. [DOI] [PubMed] [Google Scholar]
- Björklund A, Moore RY, Nobin A, Stenevi U (1973) The organization of tubero-hypophyseal and reticulo-infundibular catecholamine neuron systems in the rat brain. Brain Res 51:171–191. [DOI] [PubMed] [Google Scholar]
- Breathnach AS (1960) The cetacean central nervous system. Biol Rev 35:187–230. [Google Scholar]
- Cox B, Lee TF (1979) Evidence for an endogenous dopamine-mediated hypothermia in the rat. Br J Pharmacol 67:605–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlström A, Fuxe K (1964) Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand 62 Suppl 232:1–155. [PubMed] [Google Scholar]
- Downhower JF, Blumer LS (1988) Calculating just how small a whale can be. Nature 335:675.3173490 [Google Scholar]
- Faunt JE, Crocker AD (1987) The effects of selective dopamine receptor agonists and antagonists on body temperature in rats. Eur J Pharmacol 133:243–247. [DOI] [PubMed] [Google Scholar]
- Ferré S (1997) Adenosine-dopamine interactions in the ventral striatum. Implications for the treatment of schizophrenia. Psychopharmacology 133:107–120. [DOI] [PubMed] [Google Scholar]
- Fuxe K (1964) Cellular localization of monoamines in the median eminence and the infundibular stem of some mammals. Z Zellforsch 61:710–724. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Hökfelt T (1966) Further evidence for the existence of tubero-infundibular dopamine neurons. Acta Physiol Scand 66:245–246. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Sjöqvist F (1972) Hypothermic effect of apomorphine in the mouse. J Pharm Pharmacol 24:702–705. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Hökfelt T, Ungerstedt U (1969) Distribution of monoamines in the mammalian central nervous system by histochemical studies. In: Metabolism of Amines in the Brain. (Hooper G, ed), pp 10–22. London: Macmillan. [Google Scholar]
- Fuxe K, Hökfelt T, Ungerstedt U (1970) Morphological and functional aspects of central monoamine neurons. In: International Review of Neurobiology, Vol 13. pp 93–126. New York: Academic Press. [Google Scholar]
- Gallyas F (1979) Silver staining of myelin by means of physical development. Neurolog Res 1:203–209. [DOI] [PubMed] [Google Scholar]
- Gierlich N (1916) Zur vergleichenden Anatomie der aus dem Grosshirn stammenden Faserung. 3. Der Anteil des Cerebellum sowie der motorischen Kernlager des Hirnstammes und des Rückenmarks an dem pes pedunculi bei Phocaena und Delphinus delphis. Anat Anz 49:285–288. [Google Scholar]
- Glezer II, Jacobs MS, Morgane PJ (1988) Implications of the ‘initial brain’ concept for brain evolution in Cetacea. Behav Brain Sci 11:75–116. [Google Scholar]
- Goley PD (1999) Behavioral aspects of sleep in Pacific white-sided dolphins (Lagenorhynchus obliquidens, Gill 1865). Marine Mammal Sci 15:1054–1064. [Google Scholar]
- Gould SJ (2002) The Structure of Evolutionary Theory. Cambridge MA: Belknap Harvard. [Google Scholar]
- Hardman CD, Henderson JM, Finkelstein DI, Horne MK, Paxinos G, Halliday GM (2002) Comparison of the basal ganglia in rats, marmosets, macaques, baboons, and humans: volume and neuronal number for the output, internal relay, and striatal modulating nuclei. J Comp Neurol 445:238–255. [DOI] [PubMed] [Google Scholar]
- Hepburn D, Waterston D (1904) A comparative study of the grey and white matter of the motor-cell groups, and of the spinal accessory nerve, in the spinal cord of the porpoise (Phocaena communis). J Anat Physiol 38:105–118 & 295–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman LM, Abichandani SL, Elhajj AN, Herman EYK, Sanchez JL, Pack AA (1999) Dolphins (Tursiops truncatus) comprehend the referential character of the human pointing gesture. J Comp Psych 113:347–364. [DOI] [PubMed] [Google Scholar]
- Herman LM, Matus DS, Herman EYK, Ivancic M, Pack AA (2001) The bottlenosed dolpin’s (Tursiops truncatus) understanding of gestures as symbolic representations of its body parts. Animal Learn Behav 29:250–264. [Google Scholar]
- Hökfelt T, Johansson O, Fuxe K, Goldstein M, Park D (1976) Immunohistochemical studies on the localization and distribution of monoamine neuron system in rat brain stem. I. Tyrosine hydroxylase in the mes- and diencephalon. Med Biol 54:427–453. [PubMed] [Google Scholar]
- Hökfelt T, Martenson R, Björklund A, Kleinau S, Goldstein M (1984) Distributional maps of tyrosine-hydroxylase-immunoreactive neurons in the rat brain. In: Handbook of Chemical Neuroanatomy. Vol. 2. Classical Neurotransmitters in the CNS, part 1 (Björklund A, Hökfelt T, eds), pp 277–379. Amsterdam: Elsevier. [Google Scholar]
- Hökfelt T, Phillipson O, Goldstein M (1979) Evidence for a dopaminergic pathway in the rat descending from the A11 cell group to the spinal cord. Acta Physiol Scand 107:393–395. [DOI] [PubMed] [Google Scholar]
- Kesarev VS (1971) The inferior brain of the dolphin. Sov Sci Rev 1:52–58. [Google Scholar]
- Kesarev VS, Malofeyeva LI, Trykova OV (1977) Ecological specificity of cetacean neocortex. J Hirnforsch 18:447–460. [PubMed] [Google Scholar]
- Kitahama K, Nagatsu I, Pearson J (1994) Catecholamine systems in mammalian midbrain and hindbrain: theme and variations. In: Phylogeny and Development of Catecholamine Systems in the CNS of Vertebrates (Smeets WJAJ, Reiner A, eds), pp 183–205. Cambridge UK: Cambridge University Press. [Google Scholar]
- Kojima T (1951) On the brain of the Sperm whale (Physeter catadon). Sci Rep Whales Res Inst Tokyo 6:49–72. [Google Scholar]
- Kruger L (1959) The thalamus of the dolphin (Tursiops truncatus) and comparison with other mammals. J Comp Neurol 111:133–194. [Google Scholar]
- Kruger L (1966) Specialized features of the cetacean brain. In: Whales, Dolphins, and Porpoises (Norris KS, ed), pp 232–254. Los Angeles: University of California Press. [Google Scholar]
- Ladygina TF, Mass AM, Supin AI (1978) Multiple sensory projections in the dolphin cerebral cortex. Zh Vyssh Nerv Deiat 18:1047–1054. [PubMed] [Google Scholar]
- Lee TF, Mora F, Myers RD (1985) Dopamine and thermoregulation: An evaluation with special reference to dopaminergic pathways. Neurosci Biobehav Rev 9:589–598. [DOI] [PubMed] [Google Scholar]
- Lende RA, Akdikmen MD (1968) Motor field in cerebral cortex of the bottlenose dolphin. J Neurosurg 29:495–499. [Google Scholar]
- Lende RA, Welker WI (1972) An unusual sensory area in the cerebral neocortex of the bottlenose dolphin, Tursiops truncatus. Brain Res 45:555560. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Björklund A (1974a) The organization of the ascending catecholamine neuron systems in the rat brain as revealed by the glyoxylic acid fluorescence method. Acta Physiol Scand 92:1–48. [PubMed] [Google Scholar]
- Lindvall O, Björklund A (1974b) The glyoxylic acid fluorescence histochemical method: a detailed account of the methodology for the visualization of central catecholamine neurons. Histochemistry 39:97–127. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Björklund A (1978) Organization of catecholamine neurons in the rat nervous system. In: Handbook of Psychopharmacology, Vol. 9. (Iversen LL, Iversen SD, Snyder SH, eds), pp 139–231. New York: Plenum Publishing Corporation. [Google Scholar]
- Lindvall O, Björklund A, Skagerberg G (1983) Dopamine-containing neurons in the spinal cord – anatomy and some functional aspects. Ann Neurol 14:255–260. [DOI] [PubMed] [Google Scholar]
- Lu J, Xu M, Saper CB (2002) Identification of wake-active dopaminergic neurons in the ventral periaqueductal gray. Sleep 25:A290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyamin OI, Mukhametov LM, Siegel JM, Nazarenko JM, Polyakova IG, Shpak OV (2001) Correlation between ‘unihemispheric’ slow wave sleep and the state of eyes in a beluga whale. Sleep 24:A40–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manger PR, Fahringer HM, Pettigrew JD, Siegel JM (2002) The distribution and morphological characteristics of catecholaminergic cells in the brain of monotremes as revealed by tyrosine hydroxylase immunohistochemistry. Brain Behav Evol 60:298–314. [DOI] [PubMed] [Google Scholar]
- Manger PR, Ridgway SH, Siegel JM (2003) The locus coeruleus complex of the bottlenose dolphin (Tursiops truncatus) as revealed by tyrosine hydroxylase immunohistochemistry. J Sleep Res 12:149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino L (1998) A comparison of encephalization between odontocete cetaceans and anthropoid primates. Brain Behav Evol 51:230–238. [DOI] [PubMed] [Google Scholar]
- Marino L (2002) Convergence of complex cognitive abilities in cetaceans and primates. Brain Behav Evol 59:21–32. [DOI] [PubMed] [Google Scholar]
- Marino L, Rilling JK, Lin SK, Ridgway SH (2000a) Relative volume of the cerebellum in dolphins and comparison with Anthropoid primates. Brain Behav Evol 56:204–211. [DOI] [PubMed] [Google Scholar]
- Marino L, Uhen MD, Frohlich B, Aldag JM, Blane C, Bohaska D, Whitmore FC (2000b) Endocranial volume of mid-late Eocene Archaeocetes (Order: Cetacea) revealed by computed tomography: Implications for cetacean brain evolution. J Mammal Evol 7:81–94. [Google Scholar]
- McCowan B, Marino L, Vance E, Walke L, Reiss D (2000) Bubble ring play of bottlenose dolphins (Tursiops truncatus): implications for cognition. J Comp Psychol 114:98–106. [DOI] [PubMed] [Google Scholar]
- Mignot E, Tahiri S, Nishino S (2002) Sleeping with the hypothalamus: emerging therapeutic targets for sleep disorders. Nat Neurosci 5:1071–1075. [DOI] [PubMed] [Google Scholar]
- Morgane PJ, McFarland WL, Jacobs MS (1982) The limbic lobe of the dolphin brain: a quantitative cytoarchitectonic study. J Hirnforsch 23: 465–552. [PubMed] [Google Scholar]
- Mukhametov LM (1984) Sleep in marine mammals. Exp Brain Res Suppl 8:227–238. [Google Scholar]
- Mukhametov LM, Supin AY, Polyakova IG (1977) Interhemispheric asymmetry of the electro-encephalographic sleep patterns in dolphins. Brain Res 134:581–584. [DOI] [PubMed] [Google Scholar]
- Parada MA, de Parada MP, Rada P, Hernandez L (1995) Sulpiride increases and dopamine decreases intracranial temperature in rats when injected in the lateral hypothalamus: an animal model for the neuroleptic malignant syndrome? Brain Res 674:117–121. [DOI] [PubMed] [Google Scholar]
- Previc FH (1999) Dopamine and the origins of human intelligence. Brain Cogn 41:299–350. [DOI] [PubMed] [Google Scholar]
- Reiss D, Marino L (2001) Mirror self-recognition in the bottlenose dolphin: a case of cognitive convergence. Proc Natl Acad Sci USA 98: 5973–5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JE, Wells RS, Eide SD (2001) The Bottlenose Dolphin: Biology and Conservation. Gainesville, FL: University Press of Florida. [Google Scholar]
- Ridgway SH (1972) Homeostasis in the aquatic environment. In: Mammals of the Sea: Biology and Medicine (Ridgway SH, ed), pp 590–747. Springfield: Charles C. Thomas. [Google Scholar]
- Ridgway SH (1990) The central nervous system of the bottlenose dolphin. In: The Bottlenose Dolphin. pp 69–97. New York: Academic Press. [Google Scholar]
- Ridgway SH (2000) The auditory central nervous system of dolphins. In: Hearing in Whales and Dolphins (Au WWL, Popper AN, Fay RR, eds), pp 273–293. New York: Springer Verlag. [Google Scholar]
- Ridgway SH (2002) Asymmetry and symmetry in brain waves from dolphin left and right hemispheres: some observations after anesthesia, during quiescent hanging behavior, and during visual obstruction. Brain Behav Evol 60:265–274. [DOI] [PubMed] [Google Scholar]
- Ridgway SH, Au WWL (1999) Hearing and echolocation: Dolphin. In: Encyclopedia of Neuroscience. 2nd Edition. (Adelman G, Smith B, eds), pp 858–862. New York: Springer-Verlag. [Google Scholar]
- Salmi P, Jiminez P, Ahlenius S (1993) Evidence for specific involvement of dopamine D1 and D2 receptors in the regulation of body temperature in the rat. Eur J Pharmacol 236:395–400. [DOI] [PubMed] [Google Scholar]
- Schusterman R, Thomas J, Wood F (1986) Dolphin Cognition and Behavior: A Comparative Approach. Hillsdale NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Serafetinides EA, Shurley JT, Brooks RE (1970) Electroencephalogram of the pilot whale, Globicephala scammoni, in wakefulness and sleep: Lateralization aspects. Int J Psychobiol 2:129–133. [Google Scholar]
- Smeets WJAJ, Reiner A (1994) Phylogeny and Development of Catecholamine Systems in the CNS of Vertebrates. Cambridge UK: Cambridge University Press. [Google Scholar]
- Smeets WJAJ, González A (2000) Catecholamine systems in the brain of vertebrates: new perspectives through a comparative approach. Brain Res Rev 33:308–379. [DOI] [PubMed] [Google Scholar]
- Tillet Y (1994) Catecholamine neuronal systems in the diencephalon of mammals. In: Phylogeny and Development of Catecholamine Systems in the CNS of Vertebrates (Smeets WJAJ, Reiner A, eds), pp 207–246. Cambridge UK: Cambridge University Press. [Google Scholar]
- Ungerstedt U (1971) Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiol Scand 367:1–48. [DOI] [PubMed] [Google Scholar]


