Abstract
Three new species of Xylaria on fallen leaves in Hainan Province of China are described and illustrated, based on morphological and molecular evidence. Xylariahedyosmicola is found on fallen leaves of Hedyosmumorientale and featured by thread-like stromata with a long sterile filiform apex. Phylogenetically, X.hedyosmicola is closely related to an undescribed Xylaria sp. from Hawaii Island, USA and morphologically similar to X.vagans. Xylarialindericola is found on fallen leaves of Linderarobusta and characterised by its subglobose stromata and a long filiform stipe. It is phylogenetically closely related to X.siculaf.major. Xylariapolysporicola is found on fallen leaves of Polysporahainanensis, it is distinguished by upright or prostrate stromata and ascospores sometimes with a slimy sheath or non-cellular appendages. Xylariapolysporicola is phylogenetically closely related to X.amphithele and X.ficicola. An identification key to the ten species on fallen leaves in China is given.
Keywords: Folicolous fungi, Phylogeny, Pyrenomycetes, Taxonomy
Introduction
Species of Xylaria Hill ex Schrank are commonly found throughout the temperate, subtropical and tropical regions of the world, associated with wood, fallen fruits or seeds, fallen leaves or petioles and termite nests (Dennis 1956; Rogers 1986; Rogers and Samuels 1986; San Martin and Rogers 1989; Ju and Rogers 1999; Ju and Hsieh 2007; Fournier 2014). Previous studies on Xylaria have dealt primarily with species growing on wood and termite nests (Rogers et al. 2005; Ju and Hsieh 2007; Fournier et al. 2020), but the species diversity and distribution of the genus on other substrates, such as fallen fruits or seeds and fallen leaves or petioles, are still poorly studied (Hsieh et al. 2010; Ju et al. 2018). Especially, the study of Xylaria species growing on fallen leaves or petioles is far behind those mentioned taxa associated with other substrates and only seven species have been reported on those substrates in China (Dennis 1956; Rogers et al. 1988; Zhu and Guo 2011; Huang et al. 2014, 2015; Ma and Li 2018).
Hainan Province (20°01.04'N, 110°20.95'E) is located in southern China and enjoys a tropical monsoon climate. More than 6036 plant species, 1895 genera and 243 families have been reported in the province (Yang 2015). Different kinds of tropical vegetations (e.g. Moraceae, Euphorbiaceae and Arecaceae) and rainforests are distributed over the vast territory of the province, in which abundant fungi occur (Dai et al. 2009; Dai 2012; Gao and Yang 2016; Cui et al. 2019). Two intensive surveys of xylariaceous fungi were carried out in Hainan province in 2019 and 2020 and about 400 specimens of Xylariaceae were collected. These materials have been carefully studied through both morphological and phylogenetic methods and three new species on fallen leaves were identified. The new taxa are described and illustrated, and an identification key is provided for the 10 known species of Xylaria on fallen leaves in China.
Materials and methods
Morphological studies
Voucher specimens are deposited in the Fungarium of the Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences (FCATAS), Hainan Province, China. Samples for microscopic examination were mounted in distilled water, Melzer’s reagent, India ink or 1% SDS. Microscopic features observation, measurements and photographing were performed by using a Zeiss Axio Imager A2 microscope (Göttingen, Germany) by differential interference contrast microscopy (DIG) and brightfield microscopy (BF). The photographs of stromata, perithecia and ostioles were taken with a VHX-600E stereomicroscope Keyence Corporation (Osaka Japan). The methods of collecting, preservation and identification of the specimens follow Ma and Li (2018).
DNA extraction and sequencing
A modified cetyltrimethylammonium bromide (CTAB) extraction kit (Aidlab Biotechnologies, Beijing, China) was employed for total DNA extraction from dried specimens. The ITS region was amplified with the primer pair ITS4 and ITS5 (White et al. 1990) using the following procedure: initial denaturation at 95 °C for 3 min, followed by 30 cycles of 94 °C for 40 s, 55.8 °C for 45 s and 72 °C for 1 min and a final extension of 72 °C for 10 min. The TUB and RPB2 gene region were amplified with primers T1/T22 (O’Donnell and Cigelnik 1997) and fRPB2-5F/fRPB2-7CR (Liu et al. 1999), respectively, using the following procedure: initial denaturation at 95 °C for 3 min, followed by 35 °C cycles of 94 °C for 1 min, 52 °C for 1 min and 72 °C for 1.5 min and a final extension of 72 °C for 10 min (Hsieh et al. 2005). DNA sequencing was performed at BGI tech (Guangzhou, China) and sequences were deposited in GenBank (Table 1).
Table 1.
Species, specimens and GenBank accession number of sequences used in this study. New species and sequences are set in bold.
| Taxon | Substrate / Origin | Specimen No. | GenBank No. | Reference | ||
|---|---|---|---|---|---|---|
| ITS | TUB | RPB2 | ||||
| Xylariaacuminatilongissima | termite nests / China Taiwan | HAST 623 | EU178738 | GQ502711 | GQ853028 | Hsieh et al. (2010) |
| X.adscendens | wood / Guadeloupe | HAST 570 | GU300101 | GQ487708 | GQ844817 | Hsieh et al. (2010) |
| X.allantoidea | trunk / China Taiwan | HAST 94042903 | GU324743 | GQ502692 | GQ848356 | Hsieh et al. (2010) |
| X.amphithele | dead leaves / Guadeloupe | HAST 529 | GU300083 | GQ478218 | GQ844796 | Hsieh et al. (2010) |
| X.apoda | bark / China Taiwan | HAST 90080804 | GU322437 | GQ495930 | GQ844823 | Hsieh et al. (2010) |
| X.arbuscula | bark / China Taiwan | HAST 89041211 | GU300090 | GQ478226 | GQ844805 | Hsieh et al. (2010) |
| X.arbusculavar.plenofissura. | wood / China Taiwan | HAST 93082814 | GU339495 | GQ478225 | GQ844804 | Hsieh et al. (2010) |
| X.atrodivaricata | termite nests / China Taiwan | HAST 95052001 | EU178739 | GQ502713 | GQ853030 | Hsieh et al. (2010) |
| X.badia | bamboo culm / China Taiwan | HAST 95070101 | GU322446 | GQ495939 | GQ844833 | Hsieh et al. (2010) |
| X.bambusicola | bamboo culm / Thailand | JDR 162 | GU300088 | GQ478223 | GQ844801 | Hsieh et al. (2010) |
| X.berteri | bark / USA | JDR 256 | GU324750 | GQ502698 | GQ848363 | Hsieh et al. (2010) |
| X.berteri | bark / China Taiwan | HAST 90112623 | GU324749 | AY951763 | GQ848362 | Hsieh et al. (2010) |
| X.betulicola | leaves of Betula / China | FCATAS 750 | MF774332 | – | – | Ma and Li (2018) |
| X.brunneovinosa | termite nests / China Taiwan | HAST 720 | EU179862 | GQ502706 | GQ853023 | Hsieh et al. (2010) |
| X.castorea | wood / New Zealand | PDD 600 | GU324751 | GQ502703 | GQ853018 | Hsieh et al. (2010) |
| X.cirrata | termite nests / China Taiwan | HAST 664 | EU179863 | GQ502707 | GQ853024 | Hsieh et al. (2010) |
| X.coccophora | wood / French | HAST 786 | GU300093 | GQ487701 | GQ844809 | Hsieh et al. (2010) |
| X.crinalis | wood / China | FCATAS 751 | MF774330 | – | – | Ma and Li (2018) |
| X.crozonensis | bark / France | HAST 398 | GU324748 | GQ502697 | GQ848361 | Hsieh et al. (2010) |
| X.cubensis | log / Russian Far East | HAST 477 | – | GQ502699 | GQ848364 | Hsieh et al. (2010) |
| X.culleniae | pod / Thailand | JDR 189 | GU322442 | GQ495935 | GQ844829 | Hsieh et al. (2010) |
| X.escharoidea | termite nests / China Taiwan | HAST 658 | EU179864 | GQ502709 | GQ853026 | Hsieh et al. (2010) |
| X.feejeensis | bark / China Taiwan | HAST 92092013 | GU322454 | GQ495947 | GQ848336 | Hsieh et al. (2010) |
| X.ficicola | fallen leaves and petioles of Ficusauriculata / China | HMJAU 22818 | MZ351258 | – | – | This study |
| X.filiformis | herbaceous stem / Iran | GUM 1052 | KP218907 | – | – | Hashemi et al. (2015) |
| X.fimbriata | termite nests / French West Indies | HAST 491 | GU324753 | GQ502705 | GQ853022 | Hsieh et al. (2010) |
| X.cf.glebulosa | fruit / French West Indies | HAST 431 | GU322462 | GQ495956 | GQ848345 | Hsieh et al. (2010) |
| X.grammica | wood / China Taiwan | HAST 479 | GU300097 | GQ487704 | GQ844813 | Hsieh et al. (2010) |
| X.griseosepiacea | termite nests / China Taiwan | HAST 641 | EU179865 | GQ502714 | GQ853031 | Hsieh et al. (2010) |
| X.hedyosmicola | fallen leaves of Hedyosmumorientale / China Hainan | FCATAS 856 (HT) | MZ227121 | MZ221183 | MZ683407 | This study |
| X.hedyosmicola | fallen leaves of Hedyosmumorientale / China Hainan | FCATAS 857 | MZ227023 | MZ221184 | MZ851780 | This study |
| X.hypoxylon | wood / Belgium | HAST 152 | GU300096 | GQ260187 | GQ844812 | Hsieh et al. (2010) |
| X.hypoxylon | wood / China Taiwan | HAST 95082001 | GU300095 | GQ487703 | GQ844811 | Hsieh et al. (2010) |
| X.hypoxylon | leaf debris / Sweden | CBS 122617 | AM993146 | – | – | Persoh et al. (2009) |
| X.ianthinovelutina | fruit of Swietenia / Martinique | HAST 553 | GU322441 | GQ495934 | GQ844828 | Hsieh et al. (2010) |
| X.intraflava | termite nests / China Taiwan | HAST 725 | EU179866 | GQ502718 | GQ853035 | Hsieh et al. (2010) |
| X.juruensis | Arengaengleri / China Taiwan | HAST 92042501 | GU322439 | GQ495932 | GQ844825 | Hsieh et al. (2010) |
| X.laevis | wood / Martinique | HAST 419 | GU324746 | GQ502695 | GQ848359 | Hsieh et al. (2010) |
| X.leavis | bark / China Taiwan | HAST 95072910 | GU324747 | GQ502696 | GQ848360 | Hsieh et al. (2010) |
| X.lindericola | fallen leaves of Linderarobusta / China Hainan | FCATAS 852 (HT) | MZ005635 | MZ031978 | MZ031982 | This study |
| X.lindericola | fallen leaves of Linderarobusta / China Hainan | FCATAS 853 | MZ005636 | MZ031979 | MZ048749 | This study |
| X.liquidambar | fruits of Liquidambarformosana / China Taiwan | HAST 93090701 | GU300094 | GQ487702 | GQ844810 | Hsieh et al. (2010) |
| X.longissima | wood / China | FCATAS 749 | MF774331 | – | – | Ma and Li (2018) |
| X.longissima | wood / Iran | IRAN 16582 F | KP218906 | – | – | Hashemi et al. (2015) |
| X.meliacearum | petioles and infructescence of Guareaguidonia / Puerto Rico | JDR 148 | GU300084 | GQ478219 | GQ844797 | Hsieh et al. (2010) |
| X.multiplex | wood / USA | JDR 259 | GU300099 | GQ487706 | GQ844815 | Hsieh et al. (2010) |
| X.muscula | dead branch / French West | HAST 520 | GU300087 | GQ478222 | GQ844800 | Hsieh et al. (2010) |
| X.nigripes | termite nests / China Taiwan | HAST 653 | GU324755 | GQ502710 | GQ853027 | Hsieh et al. (2010) |
| X.oxyacanthae | fallen seeds / USA | JDR 859 | GU322434 | GQ495927 | GQ844820 | Hsieh et al. (2010) |
| X.oxyacanthae | fruits / Germany | LZ 2010-502 | HQ414587 | – | – | Roensch et al. (2010) |
| X.palmicola | fruits / New Zealand | PDD 604 | GU322436 | GQ495929 | GQ844822 | Hsieh et al. (2010) |
| X.phyllocharis> | dead leaves / French West | HAST 528 | GU322445 | GQ495938 | GQ844832 | Hsieh et al. (2010) |
| X.plebeja | trunk / China Taiwan | HAST 91122401 | GU324740 | GQ502689 | GQ848353 | Hsieh et al. (2010) |
| X.polymorpha | wood / USA | JDR 1012 | GU322460 | GQ495954 | GQ848343 | Hsieh et al. (2010) |
| X.polymorpha | Stump / Germany | M:M-0125909 | FM164944 | – | – | Persoh et al. (2009) |
| X.polysporicola | fallen leaves of Polysporahainanensis / China Hainan | FCATAS 848 (HT) | MZ005592 | MZ031976 | MZ031980 | This study |
| X.polysporicola | fallen leaves of Polysporahainanensis / China Hainan | FCATAS 849 | MZ005591 | MZ031977 | MZ031981 | This study |
| X.regalis | log of Ficusracemose / India | HAST 920 | GU324745 | GQ502694 | GQ848358 | Hsieh et al. (2010) |
| X.schweinitzii | bark / China Taiwan | HAST 92092023 | GU322463 | GQ495957 | GQ848346 | Hsieh et al. (2010) |
| X.siculaf.major | fallen leaves / China Taiwan | HAST 90071613 | GU300081 | GQ478216 | GQ844794 | Hsieh et al. (2010) |
| Xylaria sp. 6 | fallen leaves of Tibouchinasemidecandra / USA | JDR 258 | GU300082 | GQ478217 | GQ844795 | Hsieh et al. (2010) |
| X.striata | branch / China | HAST 304 | GU300089 | GQ478224 | GQ844803 | Hsieh et al. (2010) |
| X.tentaculata | leaf litter or wood / Korea | KA12-0530 | KM077162 | – | – | Kim et al. (2016) |
| X.tentaculata | leaf litter or wood / Korea | KA13-1324 | KM077163 | – | – | Kim et al. (2016) |
| X.tentaculata. | leaf litter or wood / Korea | KA13-1325 | KM077164 | – | – | Kim et al. (2016) |
| X.venosula | twigs / USA | HAST 94080508 | EF026149 | EF025617 | GQ844806 | Hsieh et al. (2010) |
| X.venustula | bark / China Taiwan | HAST 88113002 | GU300091 | GQ487699 | GQ844807 | Hsieh et al. (2010) |
| X.xylarioides | wood / Iran | GUM 1151 | KP218909 | – | – | Hashemi et al. (2015) |
| Hypoxylonfragiforme | bark / France | HAST 383 | JN979420 | AY951720 | – | Hsieh et al. (2005) |
| Camilleaobularia | – / Puerto Rico | ATCC 28093 | KY610384 | KX271243 | – | Wendt et al. (2018) |
Phylogenetic analyses
The molecular phylogeny was inferred from a combined dataset of ITS, TUB and RPB2 sequences. The sequences retrieved from open databases originated from Hsieh et al. (2005), Persoh et al. (2009), Hsieh et al. (2010), Roensch et al. (2010), Hashemi et al. (2015), Kim et al. (2016), Ma and Li (2018) and Wendt et al. (2018) (Table 1). Hypoxylonfragiforme (Pers.) J. Kickx f. and Camilleaobularia (Fr.) Læssøe, J.D. Rogers & Lodge were selected as outgroup taxa. Sequences were aligned using the MAFFT online (http://mafft.cbrc.jp/alignment/server/). Alignments were optimised manually in BioEdit 7.0.5.3 (Hall 1999).
A combined matrix of ITS-RPB2-TUB and ITS-exons of TUB and RPB2 were used to construct phylogenetic analysis by two methods including maximum likelihood (ML) and Bayesian Inference (BI) analysis, respectively. ML tree generation and bootstrap analyses were performed via the programme RAxML7.2.6 (Stamatakis 2006) running 1000 replicates combined with a ML search. Bayesian analysis was performed with MrBayes 3.1 (Huelsenbeck and Ronquist 2005) implementing the Markov Chain Monte Carlo (MCMC) technique and parameters predetermined by MrModeltest 2.3 (Nylander 2004).
Results
Molecular phylogeny
This study used genetic sequences of 57 species, including 69 ITS sequences, 57 TUB sequences and 54 RPB2 sequences. We applied two tree construction methods to improve the reliability of the results.
After the alignment sequence was adjusted using MAFFT, the ITS alignment, shown in BioEdit 7.0.5, consisted of 778 character positions, 2219 in the TUB alignment and 1241 in the RPB2 alignment. After curing, the constructed multigene alignment (MGA) consisted of 3138 characters (523 of which were derived from the ITS alignment, 1550 from TUB alignment, 1065 from RPB2 alignment). Of the MGA, 1354 characters were considered parsimony-informative.
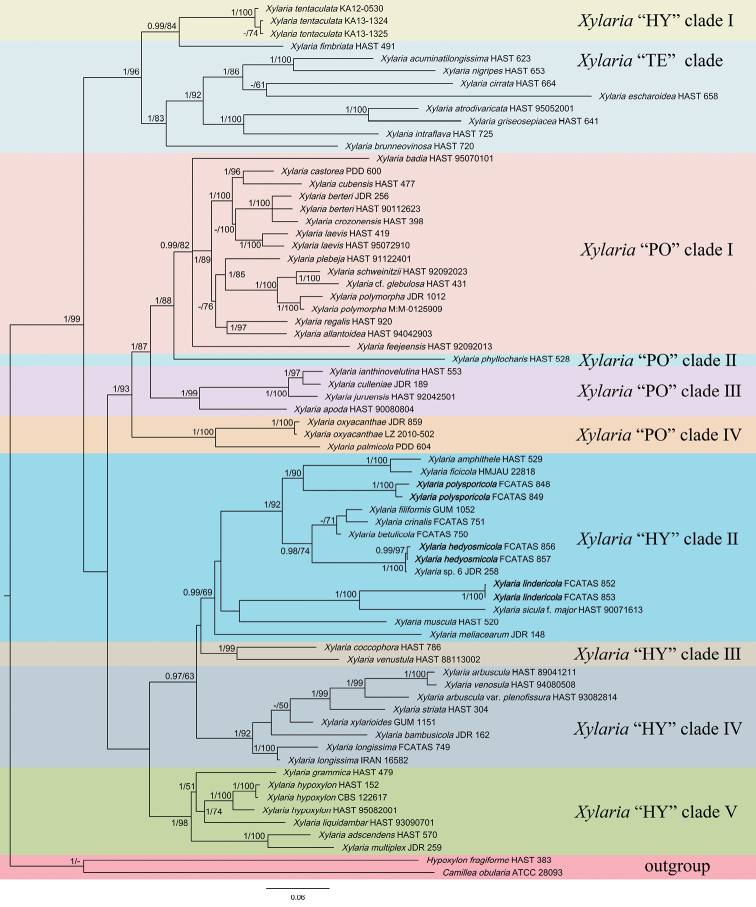
The analysis results show that the phylogenetic tree, generated by ML in RAxML7.2.6, is basically the same as that generated by BI in MrBayes 3.1. Topology of the phylogenetic analyses, based on ITS-RPB2-TUB and ITS-exons of TUB and RPB2, have no significant conflicts. Only the BI tree is shown in Figure 1 with Bayesian posterior probabilities ≥ 0.95 and ML bootstrap values ≥ 50% labelled along the branches. The phylogenetic tree showed that X.hedyosmicola is clustered with Xylaria sp. 6, X.polysporicola is clustered with X.amphithele F. San Martín & J.D. Rogers and X.ficicola Hai X. Ma, Lar.N. Vassiljeva & Yu Li, X.lindericola is clustered with X.siculaPass. & Beltr.f.major Ciccarone, but were separated from other species, as well as from each other.
Figure 1.
Phylogenetic tree of Xylaria based on multigene alignment of ITS-TUB-RPB2 in the Bayesian analysis. Bayesian posterior probabilities (≥ 0.95, before the slash markers) and RaxML bootstrap values (≥ 50, after the slash markers) are shown. Different clades are indicated as coloured blocks.
Taxonomy
. Xylaria hedyosmicola
Hai X. Ma & X.Y. Pan sp. nov.
12087348-54F1-5006-A915-68982B5B3556
839780
Figure 2.
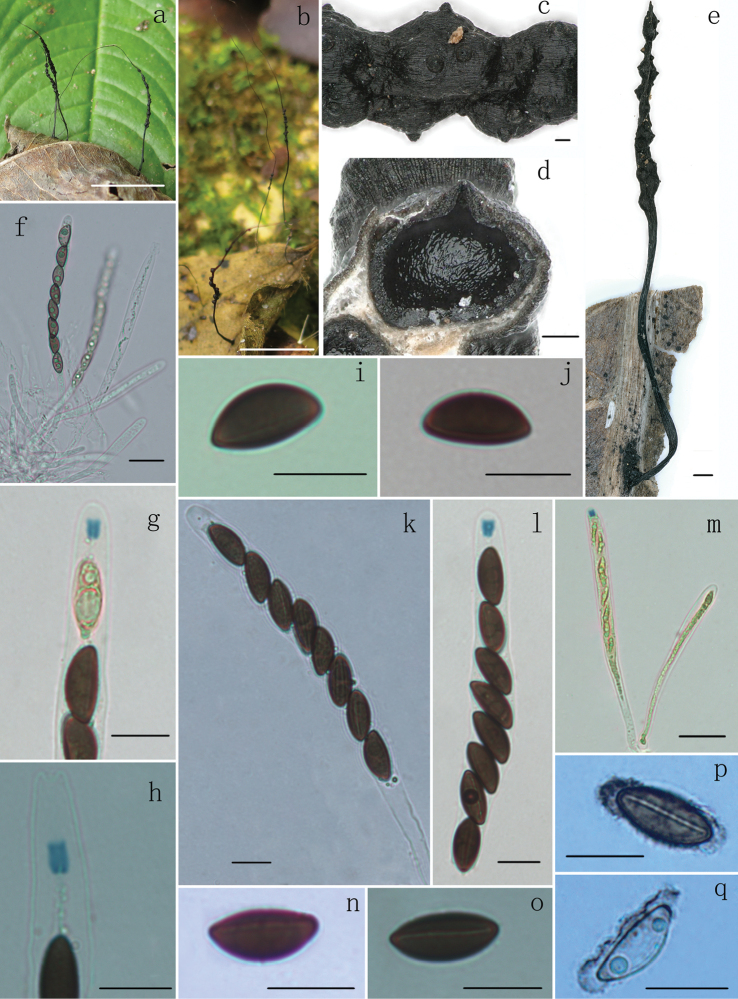
Xylariahedyosmicola (FCATAS 856, holotype) a, b, e stromata on leaves (b, FCATAS 857) c stromatal surface d section through stroma, showing a perithecium f immature asci in water g, h ascal apical ring in Melzer’s reagent i, j ascospores in Melzer’s reagent k ascus in 1% SDS l, m asci and ascal apical ring in Melzer’s reagent n ascospore in Melzer’s reagent showing straight germ slit o ascospore in Melzer’s reagent showing slightly sigmoid germ slit p, q ascospore showing a slimy sheath and non-cellular appendages in India ink. Scale bars: 1 cm (a, b); 0.1 mm (c, d); 0.5 mm (e); 20 µm (f, m); 10 µm (g–l, n–q).
Diagnosis.
Differs from X.vagans by its stromata without a black rhizomorphoid mycelium connecting dead leaves, larger ascospores and tubular to slightly urn-shaped apical apparatus. Differs from X.betulicola by its smaller stromta and larger ascospores.
Typification.
China. Hainan Province, Lingshui County, Diaoluoshan Natural Reserve, on fallen leaves of Hedyosmumorientale (Chloranthaceae), 31 December 2020, Haixia Ma (holotype, FCATAS 856).
Etymology.
“hedyosmicola” refers to the growth on leaves of Hedyosmumorientale.
Teleomorph.
Stromata upright, solitary to cespitose, thread-like, unbranched or occasionally branched once at top, 2–5.5 cm total length; with a long sterile filiform apex up to 0.5–3 cm long; fertile part 3–17 mm long × 0.5–1 mm diam., usually consisting of closely packed or scattered perithecia; stipe 8–18 mm long × 0.1–0.5 mm diam., glabrous, finely longitudinally striate, the base slightly swollen; surface roughened, with half-exposed to fully exposed perithecial contours and wrinkles. Externally black, interior white. Texture soft. Perithecia subglobose, 200–470 µm diam. Ostioles papillate, 11–22 µm diam. Asci with eight ascospores arranged in uniseriate manner, cylindrical, 105–160 µm total length, the spore-bearing parts 70–100 µm long × 8–12 µm broad, the stipes 25–70 µm long, with apical apparatus bluing in Melzer’s reagent, tubular to slightly urn-shaped, 2.5–4.8 µm high × 2.5–3.5 µm broad. Ascospores brown, unicellular, ellipsoid-inequilateral, with narrowly rounded ends, smooth, (12–)13–15(–16.7) × (6–) 6.5–7.5 (–8.5) µm (M = 14 × 7 µm, n = 60), straight to slightly sigmoid germ slit spore-length or almost spore-length, with a slimy sheath on ventral side swollen at both ends to form rounded non-cellular appendages visible in Indian ink.
Additional specimen examined.
China. Hainan Province, Lingshui County, Diaoluoshan Natural Reserve, on fallen leaves of Hedyosmumorientale, 31 December 2020, Haixia Ma (FCATAS 857).
Remarks.
Xylariahedyosmicola closely resembles X.vagans Petch by sharing thread-like or long hair-like stromata bearing closely packed or scattered perithecia with a long sterile filiform apex. Xylariavagans was originally described and illustrated by Petch (1915) from Sri Lanka. However, based on comparisons of the descriptions and illustrations, there were some differences between the two species. Xylariahedyosmicola has larger sporiferous part of asci (70–100 µm × 8–12 µm) with tubular to slightly urn-shaped apical apparatus bluing in Melzer’s reagent, brown and larger ascospores with straight (Fig. 2n and p) to slightly sigmoid germ slit (Fig. 2o), with narrowly rounded ends and a slimy sheath on ventral side swollen at both ends to form rounded non-cellular appendages, while X.vagans has a black rhizomorphoid mycelium connecting dead leaves, smaller sporiferous part 68–72 µm × 6 µm and black-brown, cymbiform, smaller ascospores 9–12 × 5–6 µm, with broadly rounded ends and is without apical apparatus, germ slit and sheath or appendages (Petch 1915). Unfortunately, the molecular sequences of X.vagans from Sri Lanka were not available.
Xylariabetulicola Hai X. Ma, Lar.N. Vassiljeva & Yu Li is similar to X.hedyosmicola in stromatal morphology, but differs in having larger stromata 3–7 cm, slightly smaller ascospores (11.5)12–14(15) × 5–6 µm, without sheath or appendages (Ma and Li 2018). In the phylogenetic tree, X.hedyosmicola formed a fully supported clade with Xylaria sp. 6 from Hawaiian Islands, USA (Hsieh et al. 2010). Although there are no descriptions on Xylaria sp. 6 in the study of Hsieh et al. (2010), we suspected that it is conspecific with X.hedyosmicola. The sequences comparison showed that there are 98.7%, 99% and 99.9% maximal percentage identities, respectively in ITS, TUB and RPB2 between X.hedyosmicola (FCATAS 856) and Xylaria sp. 6 from USA (JDR 258).
. Xylaria lindericola
Hai X. Ma & X.Y. Pan sp. nov.
8273E502-A2D0-5CBF-BBDA-8B1CA1211B59
839554
Figure 3.
Xylarialindericola (FCATAS 852, holotype) a, b stromata on leaves c fertile part of stroma d stromatal surface e section through stroma, showing perithecia f ascal apical ring and ascospores with beaked ends in Melzer’s reagent g ascus and ascal apical ring in Melzer’s reagent h ascus in water i, j ascospores in water k, l ascospore in Melzer’s reagent m ascospore in India ink n ascospore in 1% SDS showing germ slit. Scale bars: 1.5 cm (a, b); 0.2 mm (c–e); 10 µm (f–n).
Diagnosis.
Differs from X.siculaf.major by its subglobose stromata without a long sterile apex, larger ascospores and host plant. Differs from X.hypsipoda by its black stromata, glabrous stipes and smaller apical apparatus.
Typification.
China. Hainan Province, Lingshui County, Diaoluoshan Natural Reserve, on fallen leaves of Linderarobusta (Lauraceae), 31 December 2020, Haixia Ma (holotype, FCATAS 852).
Etymology.
“lindericola” refers to the growth on leaves of Linderarobusta.
Teleomorph.
Stromata upright or prostrate, solitary to cespitose, unbranched or branched once or more at stipe, 3–26 cm total length; fertile part subglobose on long filiform stipes, 0.1–0.4 cm diam., the stipe 3–25 cm long × 0.1–1 mm diam., glabrous, finely longitudinally striate, the base slightly swollen; surface roughened by wrinkles and barely exposes perithecial contours. External black, interior white. Texture soft. Perithecia subglobose, 300–550 µm diam. Ostioles black, papillate. Asci with eight ascospores in uniseriate manner, cylindrical, 105–165 µm total length, the spore-bearing parts 65–115 µm long × 7.5–10.5 µm broad, the stipes 25–65 µm long, with apical apparatus bluing in Melzer’s reagent, tubular to urn-shaped, 3.9–5.5 µm high × 3–5 µm broad. Ascospores brown, unicellular, ellipsoid-inequilateral, with slightly narrowly rounded ends, aberrant ascospores with strongly pinched or beaked ends, smooth, (12.5–)13.5–15.5(–18) × (7–) 7.5–8.5 (–9.5) µm (M = 14.8 × 8 µm, n=60), with straight germ slit spore-length, without sheath or appendages visible in India ink.
Additional specimen examined.
China. Hainan Province, Lingshui County, Diaoluoshan Natural Reserve, on fallen leaves of Linderarobusta, 31 December 2020, Haixia Ma (FCATAS 853).
Remarks.
Xylarialindericola is distinguished by its subglobose fertile part of stroma on a long filiform stipe and growing on fallen leaves of Linderarobusta. The species is somewhat similar to X.siculaf.major in morphology of stromatal fertile part. However, X.siculaf.major has stromata with long sterile apex, slightly smaller ascospores 9–13(–15) × (3–) 4.5–6 (–7) µm and grows on dead Olea leaves (Ciccarone 1947; Graniti 1959; Fournier 2014). In the phylogenetic tree, X.lindericola formed a fully supported clade with X.siculaf.major (Figure 1).
Xylariahypsipoda Massee is similar to X.lindericola by sharing globose stromata and ascospores dimensions, but differs in having stromata with whitish scales, hairy stipes and urn-shaped, slightly larger apical apparatus 5–8 µm high × 2.9–5 µm broad (Rogers et al. 1987).
Xylariaficicola resembles X.lindericola in stromatal morphology, but differs in having strongly exposed perithecial mounds of stromatal surface, larger ascospores (16–) 17.5–21(–22.7) × 6.5–8.5 µm with conspicuous hyaline noncellular appendage and grows on fallen leaves and petioles of Ficusauriculata (Ma et al. 2011). Xylariaheloidea Penz. & Sacc. from Indonesia is somewhat similar to X.lindericola in stromatal morphology, but the former has obconical, convex stromatal top, larger ascospores (14.5–) 15.5–18(–19) × (5–)5.5–6.5(–7) µm (16.7 × 6.1 µm), with a hyaline sheath swelling at both ends to form non-cellular appendages and grows on fallen fruits, twigs, petioles, and leaves of various plants (Ju et al. 2018).
Xylariacomosa (Mont.) Fr. and X.clusiae K.F. Rodrigues, J.D. Rogers & Samuels are also somewhat similar to X.lindericola in stromatal morphology. However, X.comosa has larger ascospores (21)–26–40 × 7–11 µm and larger apical ring 10.5 µm high × 7.5 µm broad (Dennis 1956) and X.clusiae has smaller stromata 1–3.5 cm, ascospores broadly ovoida1 to nearly globose (11.6–)12.8–16.7(–18) × 8–15 µm, with colorless appendage at one end (Samuels and Rogerson 1990).
. Xylaria polysporicola
Hai X. Ma & X.Y. Pan sp. nov.
697BC6F2-DC69-5FDC-BDE5-B67B0EAB0FAC
839552
Figure 4.
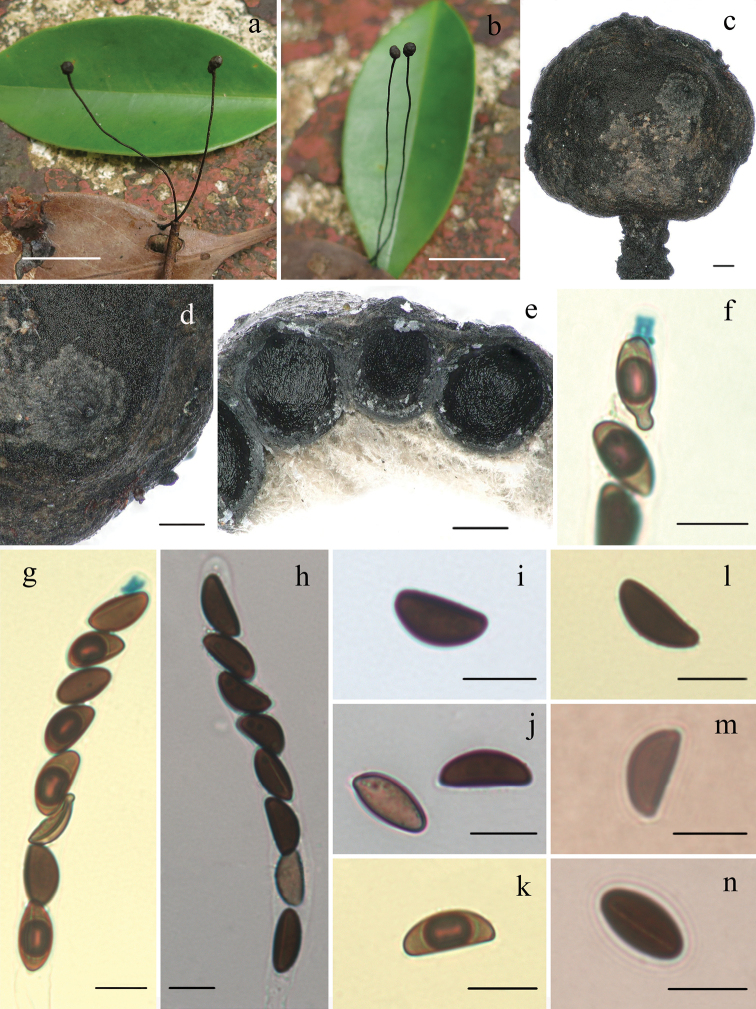
Xylariapolysporicola (FCATAS 848, holotype) a, b stromata on leaves (b, FCATAS 851) c stromatal surface d section through stroma, showing perithecia e, g asci and ascal apical ring in Melzer’s reagent f, i ascal apical ring in Melzer’s reagent h asci in black India ink j ascospore with germ slit in 1% SDS k, l ascospore in water m, n ascospore showing a slimy sheath and non-cellular appendages in India ink (FCATAS 850) o Ascospore in 1% SDS. Scale bars: 1 cm (a, b); 0.2 mm (c, d); 10 µm (e–o).
Diagnosis.
Differs from X.phyllocharis by its half-exposed to fully exposed perithecial contours, the fertile part cylindrical and larger perithecia. Differs from X.phyllophila by its smaller ascospores. Differs from X.amphithele by its cylindrical stromata.
Typification.
China. Hainan Province, Lingshui County, Diaoluoshan Natural Reserve, on fallen leaves of Polysporahainanensis (Theaceae), 31 December 2020, Haixia Ma (holotype, FCATAS 848).
Etymology.
“polysporicola” refers to the growth on leaves of Polysporahainanensis.
Teleomorph.
Stromata solitary, upright or prostrate, cylindrical, unbranched or occasionally branched, 1–4 cm total length, with acute sterile apex up to 2 mm long; fertile part 2–15 mm long × 0.5–1.6 mm diam., usually consists of closely packed perithecia and occasionally with scattered perithecia; the stipe 5–30 mm long × 0.3–1 mm diam., glabrous, finely longitudinally striate, the base slightly swollen; surface roughened, with half-exposed to fully exposed perithecial contours and wrinkles. Externally black, interior white. Texture soft. Perithecia subglobose, 0.4–0.6 mm diam. Ostioles papillate. Asci with eight ascospores arranged in uniseriate manner, cylindrical, 115–185 µm total length, the spore-bearing parts 75–100 µm long × 6.5–9 µm broad, the stipes 30–90 µm long, with apical apparatus bluing in Melzer’s reagent, inverted hat-shaped or urn-shaped, 2.5–4.5 µm high × 2–3.2 µm broad. Ascospores brown to dark-brown, unicellular, ellipsoidal-inequilateral, with broadly rounded ends, one end slightly pinched sometimes, smooth, (11.5–)12.5–14.5(–15) × 5.5–8 µm (M = 13.2 × 6.4 µm, n=60), with straight germ slit slightly less than spore-length, a slimy sheath or non-cellular appendages visible occasionally in Indian ink.
Additional specimens examined.
China. Hainan Province, Lingshui County, Diaoluoshan Natural Reserve, on fallen leaves of Polysporahainanensis, 31 December 2020, Haixia Ma (FCATAS 849); 5 July 2019, Haixia Ma (FCATAS 850 & 851).
Remarks.
Xylariapolysporicola is morphologically similar to X.phyllocharis Mont. However, X.phyllocharis has fully immersed perithecia, the fertile part with peg-like structures and smaller perithecia 0.2–0.3 mm diam (San Martín and Rogers 1989; Fournier et al. 2020). Xylariapolysporicola is similar to Xylaria sp. (80082005) from Taiwan in stromatal morphology, but the latter has slightly smaller stroma (11–14 mm total length × 1 mm diam. vs. 10–40 mm total length × 0.5–1.6 mm diam.), hard texture, slightly larger ascospores 13.5–16.5 × 5–6 µm, with narrowly rounded ends (Ju and Rogers 1999). Xylariaphyllophila Ces. somewhat resembles X.polysporicola in stromatal morphology, but the former has larger ascospores 20 × 10 µm (Cooke 1883).
Xylariapolysporicola is somewhat similar to X.amphithele F. San Martín & J.D. Rogers in shape and size of apical apparatus and ascospores. However, X.amphithele has globose to conical stromata with 3–4 to 20 naked perithecia (San Martín and Rogers 1989). In the phylogenetic tree, X.polysporicola formed a lineage close to X.amphithele and X.ficicola, but is distant from X.phyllocharis.
Discussion
We included ten Xylaria species on fallen leaves in the phylogenetic analyses of the present study. Except for X.phyllocharis, the other nine studied species formed a monophyletic clade with two wood-inhabiting species, X.muscula Lloyd and X.crinalis Hai X. Ma, Lar. N. Vassiljeva & Yu Li, in our phylogenetic tree (Figure 1). In China, only three species have been previously reported with molecular evidence: X.ficicola from tropical Yunnan, X.siculaf.major from tropical Taiwan and X.betulicola from temperate Jilin (Ma and Li 2018). Within the clade, X.meliacearum, associated with petioles and infructescence of Guareaguidonia, formed a separate branch from other Xylaria species on other leaves. In Hsieh et al. (2010), X.phyllocharis grouped with the wood-inhabiting Xylaria species, which did not reveal any contradictions in our tree. Three species, X.polysporicola, X.amphithele and X.ficicola formed a highly supported clade. Morphologically, these species have some similar features, such as ascospores with slimy sheath or non-cellular appendages, inverted hat-shaped or urn-shaped apical apparatus (San Martín and Rogers 1989; Ma et al. 2020). As Xylariahedyosmicola formed a fully supported clade with Xylaria sp. 6, the two species should be the same, based on the ITS-TUB-RPB2 (Hsieh et al. 2010). Xylarialindericola, on leaves of Linderarobusta formed a sister lineage to X.siculaf.major on unknown fallen leaves with high bootstrap value 100%. Xylariamuscula, growing on dead branches, formed a weakly supported branch with X.lindericola and X.siculaf.major associated with fallen leaves in our tree. This may be because our phylogenetic analysis did not include more taxa related to X.muscula.
Until now, ten taxa, X.betulicola, X.diminuta F. San Martín & J.D. Rogers, X.ficicola, X.foliicola G. Huang & L. Guo, X.hainanensis Y.F. Zhu & L. Guo, X.hedyosmicola, X.jiangsuensis G. Huang & L. Guo, X.lindericola, X.polysporicola and X.siculaf.major have been found on fallen leaves in China (Hsieh et al. 2010; Ma et al. 2011; Zhu and Guo 2011; Huang et al. 2014, 2015; Ma and Li 2018). Amongst these species, X.diminuta, originally reported from Mexico, was found in Yunnan province of China in 2013 (Huang et al. 2014). Xylariasiculaf.major was first described from Sicily in 1878 and then found in Spain, Kenya, Sardinia, and Taiwan province of China (Hsieh et al. 2010; Fournier 2014). Unfortunately, except for the three species in this study, the molecular data of the other Xylaria species from China were not available. We anticipate that additional species of Xylaria on fallen leaves will be discovered as more studies are conducted.
Key to species of Xylaria on fallen leaves in China
| 1 | Stromata with rounded fertile apices | 2 |
| – | Stromata with acute sterile apices | 3 |
| 2 | Stromata associated with leaves and petioles of Ficusauriculata (Moraceae), ascospores (16–)17.5–21(–22.7) × 6.5–8.5 µm | X.ficicola |
| – | Stromata associated with leaves of Linderarobusta (Lauraceae), ascospores (12.5–)13.5–15.5(–18) × (7–)7.5–8.5(–9.5) µm | X.lindericola |
| 3 | Stipes tomentose | X.hainanensis |
| – | Stipes glabrous | 4 |
| 4 | Fertile part subglobose | X.siculaf.major |
| – | Fertile part not subglobose | 5 |
| 5 | Stromata cylindrical | 6 |
| – | Stromata filiform | 8 |
| 6 | Ascospores (5.5–)6–8 × 3–3.5(–4) µm | X.diminuta |
| – | Ascospores length > 8.5 µm | 7 |
| 7 | Stromata with conspicuous perithecial contours, ascospores (11.5–)12.5–14.5(–15) × 5.5–8 µm | X.polysporicola |
| – | Stromata with inconspicuous perithecial contours, ascospores (8.5–)9–11 × 4–6 µm | X.foliicola |
| 8 | Ascospores 16.5–20(–21.5) × 4–5(–6) µm | X.jiangsuensis |
| – | Ascospores length < 16.5 µm | 9 |
| 9 | Stromata associated with leaves of Betula (Betulaceae), ascospores (11.5)12–14(15) × 5–6 µm, with a straight germ slit, without appendages visible in India ink | X.betulicola |
| – | Stromata associated with leaves of Hedyosmumorientale (Chloranthaceae), ascospores (12–)13–15(–16.7) × (6–)6.5–7.5(–8.5) µm, with straight to slightly sigmoid germ slit, with appendages visible in Indian ink | X.hedyosmicola |
Supplementary Material
Acknowledgements
The authors thank Prof. Yu-Ming Ju (Institute of Plant and Microbial Biology, Academia Sinica, Taiwan, China) for suggestions on the manuscript. This study was supported by the National Natural Science Foundation of China (no. 31770023, 31972848, U1803232). We are also grateful to the Key Research and Development Program of Hainan (ZDYF2020062) and Hainan Basic and Applied Research Project for Cultivating High-Level Talents (2019RC305).
Citation
Pan X-Y, Song Z-K, Qu Z, Liu T-D, Ma H-X (2022) Three new Xylaria species (Xylariaceae, Xylariales) on fallen leaves from Hainan Tropical Rainforest National Park. MycoKeys 86: 47–63. https://doi.org/10.3897/mycokeys.86.71623
Contributor Information
Tie-Dong Liu, Email: liu@hainanu.edu.cn.
Hai-Xia Ma, Email: mahaixia@itbb.org.cn.
References
- Ciccarone (1947) Alcune osservazioni su una forma de Xylariasicula Pass. e Beltr. Nuovo Giornale Botanico Italiano 53(1946): 356–358. [Google Scholar]
- Cooke MC. (1883) On Xylaria and its allies. Grevillea 11: 81–94. [Google Scholar]
- Cui BK, Li HJ, Ji X, Zhou JL, Song J, Si J, Yang ZL, Dai YC. (2019) Species diversity, taxonomy and phylogeny of Polyporaceae (Basidiomycota) in China. Fungal Diversity 97: 137–392. 10.1007/s13225-019-00427-4 [DOI] [Google Scholar]
- Dai YC. (2012) Polypore diversity in China with an annotated checklist of Chinese polypores. Mycoscience 53: 49–80. 10.1007/s10267-011-0134-3 [DOI] [Google Scholar]
- Dai YC, Yang ZL, Cui BK, Yu CJ, Zhou LW. (2009) Species diversity and utilization of medicinal mushrooms and fungi in China. International Journal of Medicinal Mushrooms 11: 287–302. 10.1615/IntJMedMushr.v11.i3.80 [DOI] [Google Scholar]
- Dennis RWG. (1956) Some Xylarias of tropical America. Kew Bulletin 11: 401–444. 10.2307/4109126 [DOI] [Google Scholar]
- Fournier J. (2014) Update on European species of Xylaria, 120 pp.
- Fournier J, Lechat C, Courtecuisse R. (2020) The genus Xylaria sensu lato (Xylar-iaceae) in Guadeloupe and Martinique (French West Indies) III. Taxa with sl-ender upright stromata. Ascomycete. org 12(3): 81–164. 10.1128/jb.172.8.4238-4246.1990 [DOI] [Google Scholar]
- Gao Q, Yang ZL. (2016) Diversity and distribution patterns of root-associated fungi on herbaceous plants in alpine meadows of southwestern China. Mycologia 108: 281–291. 10.3852/14-324 [DOI] [PubMed] [Google Scholar]
- Graniti (1959) Presenza di Xylariasicula Pass. Et Beltr. su frutti e foglie di olivo e considerazioni sulla specie e sulle sue forme. Nuovo Giornale Botanico Italiano 66: 364–376. [Google Scholar]
- Hall TA. (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. NucleicAcids Symposium Series 41: 95–98. 10.1021/bk-1999-0734.ch008 [DOI] [Google Scholar]
- Hashemi SA, Zare R, Khodaparast SA, Elahinia SA. (2015) A new Xylaria speciesfrom Iran. Mycologia Iranica 2: 1–10. [Google Scholar]
- Hsieh HM, Ju YM, Rogers JD. (2005) Molecular phylogeny of Hypoxylon and rel-ated genera. Mycologia 97(4): 844–865. 10.1080/15572536.2006.11832776 [DOI] [PubMed] [Google Scholar]
- Hsieh HM, Lin CR, Fang MJ, Rogers JD, Fournier J, Lechat C, Ju YM. (2010) P-hylogenetic status of Xylariasubgen.Pseudoxylaria among taxa of the subfa-mily Xylarioideae (Xylariaceae) and phylogeny of the taxa involved in the s-ubfamily. Molecular Phylogenetics and Evolution 54: 957–969. 10.1016/j.ympev.2009.12.015 [DOI] [PubMed] [Google Scholar]
- Huang G, Guo L, Liu N. (2014) Two new species of Xylaria and X.diminuta ne-w to China. Mycotaxon 129(1): 149–152. 10.5248/129.149 [DOI] [Google Scholar]
- Huang G, Wang RS, Guo L, Liu N. (2015) Three new species of Xylaria from China. Mycotaxon 130: 299–304. 10.5248/130.299 [DOI] [Google Scholar]
- Huelsenbeck JP, Ronquist F. (2005) Bayesian analysis of molecular evolution using MrBayes. In: Nielsen R. (Ed.) Statistical methods in molecular evolution.Springer, New York, 183–226. 10.1007/0-387-27733-1_7 [DOI]
- Ju YM, Rogers JD. (1999) The Xylariaceae of Taiwan (excluding Anthostomella). Mycotaxon 73: 343–440. [Google Scholar]
- Ju YM, Hsieh HM. (2007) Xylaria species associated with nests of Odontotermesf-ormosanus in Taiwan. Mycologia 99: 936–957. 10.1080/15572536.2007.11832525 [DOI] [PubMed] [Google Scholar]
- Ju YM, Rogers JD, Hsieh HM. (2018) Xylaria species associated with fallen fruitsand seeds. Mycologia 110(4): 726–749. 10.1080/00275514.2018.1469879 [DOI] [PubMed] [Google Scholar]
- Kim CS, Jo GW, Kwag YN, Oh SO, Lee SG, Sung GH, Oh G, Shrestha B, Ki-m SY, Shin CH, Han SK. (2016) New Records of Xylaria Species in Korea: X.ripicola sp. nov. and X.tentaculata. Mycobiology 44(1): 21–28. 10.5941/MYCO.2016.44.1.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laessoe T, Lodge DJ. (1994) Three host-specific Xylaria species. Mycologia 86(3): 436–446. 10.1080/00275514.1994.12026431 [DOI] [Google Scholar]
- Liu YJ, Whelen S, Hall BD. (1999) Phylogenetic relationships among ascomycetes: evidence from an RNA polymerse II subunit. Molecular Biology And Evolution 16: 1799–1808. 10.1093/oxfordjournals.molbev.a026092 [DOI] [PubMed] [Google Scholar]
- Ma HX, Li Y. (2018) Xylariacrinalis and X.betulicola from China – two new s-pecies with thread-like stromata. Sydowia 70: 37–49. 10.12905/0380.sydowia70-2018-0037 [DOI] [Google Scholar]
- Ma HX, Vasilyeva L, Li Y. (2011) A new species of Xylaria from China. Mycotaxon 116: 151–155. 10.5248/116.151 [DOI] [Google Scholar]
- Ma HX, Qu Z, Peng MK, Li Y. (2020) Two penzigioid Xylaria species described from China based on morphological and molecular characters. Phytotaxa 436(1): 036–044. 10.11646/phytotaxa.436.1.3 [DOI] [Google Scholar]
- Nylander JAA. (2004) MrModeltest 2.3. Program distributed by the author. Evolutionary Biology Center, Uppsala University.
- O’Donnell K, Cigelnik E. (1997) Two Divergent Intragenomic rDNA ITS2 Types within a Monophyletic Lineage of the Fungus. Molecular Phylogenetics and Evolution 7(1): 103–116. 10.1006/mpev.1996.0376 [DOI] [PubMed] [Google Scholar]
- Persoh D, Melcher M, Graf K, Fournier J, Stadler M, Rambold G. (2009) Molecu-lar and morphological evidence for the delimitation of Xylariahypoxylon. M-ycologia 101(2): 256–268. 10.3852/08-108 [DOI] [PubMed] [Google Scholar]
- Petch (1915) Annals of the Royal Botanical Gardens, Peradeniya 6(1): e68.
- Roensch P, Roensch S, Reiher A, Otto P. (2010) Investigations on the fructicolous Xylariadelitschii and Xylaria Oxyacanthae. Boletus 32(2): 106–122. [Google Scholar]
- Rogers JD. (1986) Provisional keys to Xylaria species in continental United States. Mycotaxon 26: 85–97. [Google Scholar]
- Rogers JD, Samuels GJ. (1986) Ascomycetes of New Zealand 8. Xylaria. New Ze-aland Journal of Botany 24(4): 615–650. 10.1080/0028825X.1986.10409947 [DOI] [Google Scholar]
- Rogers DJ, Callan BE, Samuels GJ. (1987) The Xylariaceae of the rain forests of North Sulawesi (Indonesia). Mycotaxon 31: 113–172. [Google Scholar]
- Rogers JD, Ju YM, Lehmann J. (2005) Some Xylaria species on termite nests. Mycologia 97(4): 914–923. 10.1080/15572536.2006.11832783 [DOI] [PubMed] [Google Scholar]
- Rogers JD, Callan BE, Rossman AY, Samuels GJ. (1988) Xylaria (Sphaeriales, Xylariaceae) from Cerro de la Neblina, Venezuela. Mycotaxon 31: 103–153. 10.1007/BF00455669 [DOI] [Google Scholar]
- Samuels GJ, Rogerson CT. (1990) New Ascomycetes from the Guayana Highland. Memoirs of the New York Botanical Garden 64: 165–183. [Google Scholar]
- San Martin GF, Rogers JD. (1989) A preliminary account of Xylaria of Mexico. Mycotaxon 34: 283–373. [Google Scholar]
- Stamatakis A. (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. 10.1093/bioinformatics/btl446 [DOI] [PubMed] [Google Scholar]
- Wendt LC, Sir EB, Kuhnert E, Heitkaemper S, Lambert C, Hladki AI, Romero A-I, Luangsa-ard JJ, Srikitikulchai P, Persoh D, Stadler M. (2018) Resurrection and emendation of the Hypoxylaceae, recognised from amultigene phylogeny of the Xylariales. Mycological Progress 17: 115–154. 10.1007/s11557-017-1311-3. [DOI] [Google Scholar]
- White TJ, Burns T, Lee S, Taylor J. (1990) Amplification and direct sequencing o-f fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (Eds) PCR protocols, a guide to methods andapplications.Academic, San Diego, 315–322. 10.1016/B978-0-12-372180-8.50042-1 [DOI]
- Yang XB. (2015) The colored illustrated flora of Hainan Province. Science Press, Beijing. [in Chinese]
- Zhu YF, Guo L. (2011) Xylariahainanensis sp. nov. (Xylariaceae) from China. M-ycosystema 30(4): 526–528. 10.13346/j.mycosystema.2011.04.014 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.