Figure 4. OCT4 and Nanog gRNA inhibit TGF-β1-induced NTCP-Huh7.5.1 and LX2 cell migratory capacity.
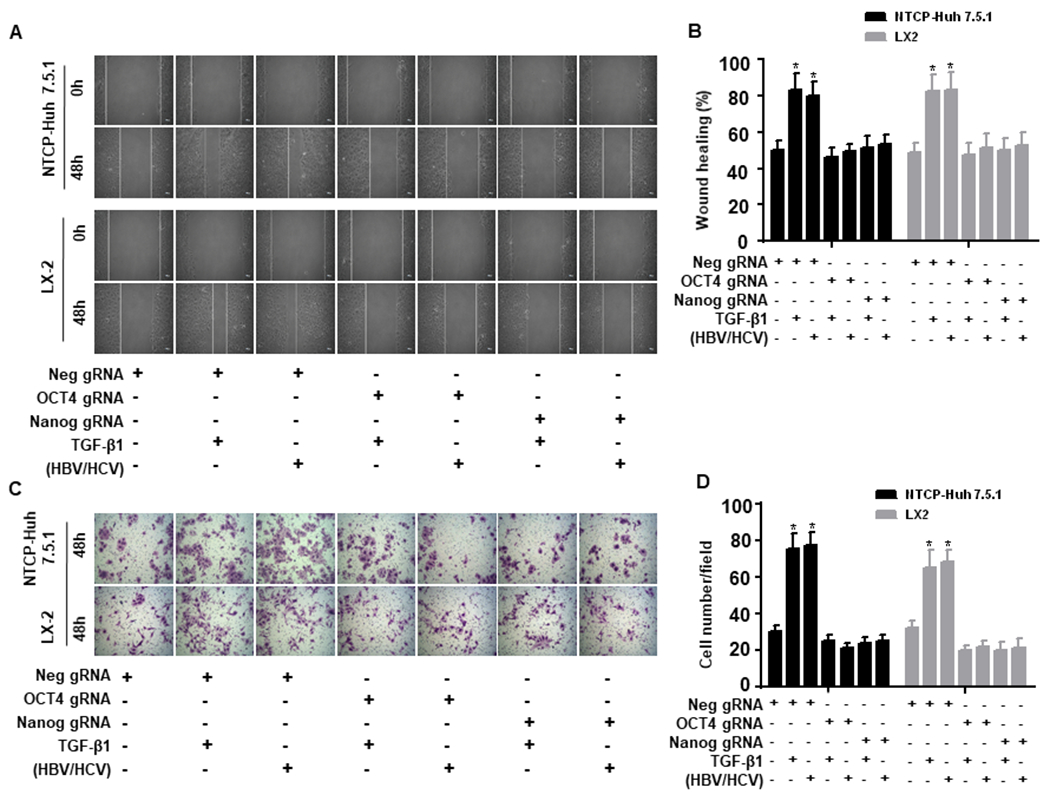
NTCP-Huh7.5.1 or LX2 (100,000 cell/well in 2 mL) cells were seeded into 6 well-plates and transfected with Neg gRNA, OCT4 gRNA or Nanog gRNA for overnight. The cell monolayer was scratched using a sterilized P200 pipette tip. Cells were washed with PBS and maintained in 2 mL fresh DMEM with 10% FBS. 100 μL of HBVvp and 100 μL HCVvp were added to the appropriate NTCP-Huh7.5.1 wells, 100 μL of HBVs and 100 μL HCVs were added to the appropriate LX2 wells, 200 μL of uninfected supernatant or TGF-β1(100 ng/mL) was added to the appropriate well for 48 hours.
(A). Images of NTCP-Huh7.5.1 (top) and LX-2 cell (bottom) scratch assays at 0 and 48 hours.
(B). Proportion of migrated NTCP-Huh7.5.1 or LX2 cells.
(C). NTCP-Huh7.5.1 or LX2 cells (100,000 cell/well in 2 mL) were seeded into 6 well-plates and transfected with empty gRNA, OCT4 gRNA or Nanog gRNA for overnight. The treated cells were harvested and seeded onto the up chamber (50,000 cell/well in 2 mL) of a 12 well Corning transwell plate (with 8 μm transmembrane pore). 100 μL of HBVvp and 100 μL of HCVvp were added to the appropriate NTCP-Huh7.5.1 cells, 100 μL of HBVs and 100 μL of HCVs were added to the appropriate LX2 cells, 200 μL of uninfected supernatant or TGF-β1(100 ng/mL) was added to the appropriate well for 48 hours. Cells that migrated to the bottom chamber were washed three times with PBS, fixed with 4% paraformaldehyde and stained with 0.05% crystal violet. Cells in the bottom chamber were counted using an inverted light microscope (200 X). (D) Number of migrated NTCP-Huh7.5.1 or LX2 cells. Data are representative of 3 independent experiments with similar results. Bars represent means ± SD of 3 biological repeats. *, p < 0.05. **, p < 0.01.
