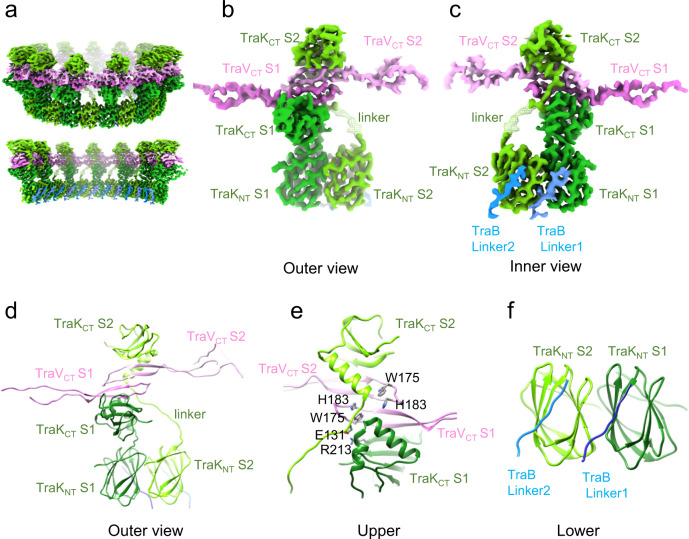
Fig. 2. Structure of the outer ring complex (ORC).
a Overview (upper) and a central cut view (bottom) of the 3.31 Å ORC from the pED208-carrying (WT) strain reconstructed with 13-fold symmetry imposed. b, c Outer and inner views of the heterodimer structural unit including two TraK monomers and two C-terminal domains of TraV (TraVCT). Different subunits are denoted S1 and S2. The two N-terminal domains of TraK (TraKNT) are arranged side-by-side, and the two C-terminal domains (TraKCT) are flipped 180° relative to each other. The two TraVCT domains pack antiparallel to each other. A flexible linker connecting the TraKNT-S2 and TraKCT-S2 domains is visible as a blurred density at low (10 Å) resolution. Two TraB linker segments corresponding to residues R176-186 form specific contacts with TraKNT domains. d,e,f Atomic models of the (d) entire structural unit, (e) the upper TraKCT/TraVCT complex showing residues whose interactions are predicted to stabilize the ORC, and (f) the lower TraKNT dimer with associated TraB linker segments. Colors for all panels: TraK-S1 and TraV-S2(dark and light green), TraV-S1 and TraV-S2 (dark and light pink), TraBR176-S186 linkers (blue).