Fig. 3. Structure of central cone (CC).
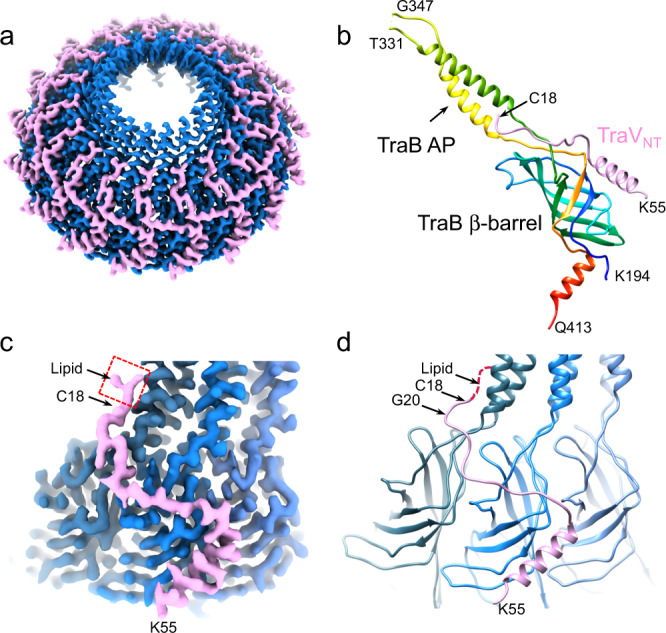
a Overview of the 2.95 Å CC from the pED208-carrying (WT) strain reconstructed with 17-fold symmetry imposed. The CC is composed of domains of TraB (blue) and TraV (pink). b The atomic model of the β-barrel and AP domains of TraB (residues K194-Q413) in rainbow color, with the N-terminal domain of TraV (TraVNT, residues C18-K55) (pink). An AP flexible loop (residues T332-I346) cannot be built in the model. c, d Interaction between the TraVNT domain and three adjacent TraBβ-barrel domains visualized in densities and models, respectively. The red box in (c) shows a piece of lipid density connected to TraV C18.
