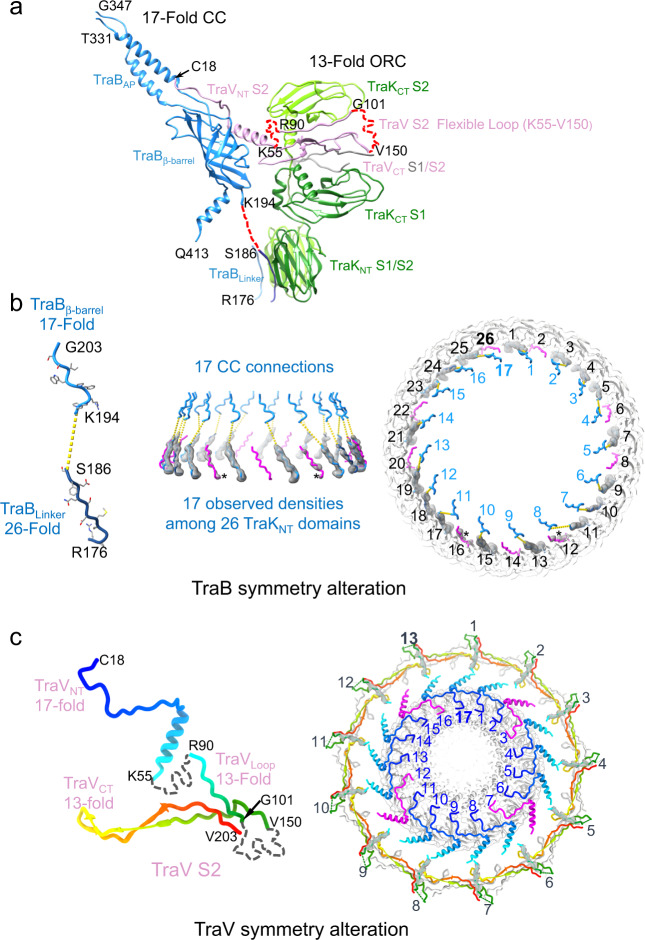
Fig. 4. TraV and TraB accommodate the C13:C17 symmetry mismatch.
a An asymmetric unit of the OMCCF. Thirteen TraK/TraVCT units comprise the C13 ORC, while the 17 TraBβ-barrel/TraVNT units assemble as the C17 CC. TraV and TraB bridge the ORC and CC substructures through flexible loops (red dash lines). Subunit domains and colors are shown as described in Fig. 2 legend. Residues shown denote boundaries of the different domains. A short segment (residues R90-G101) in the TraV S2 flexible linker forms a traceable contact with the TraKCT S2 domain. Two TraBR176-S186 linker segments are shown to denote detectable contacts with both S1 and S2 domains of the TraKNT dimer. b Left: Segment of TraB involved in the intraprotein symmetry alteration. Residues K194-G203 are part of the TraBβ-barrel domain, 17 copies of which form the CC. Residues S186-K194 bridge the symmetry mismatch, but can be deleted without affecting T4SSF function. Residues R176-S186 bind a subset of the 26 copies of TraKNT domains comprising the ORC. Middle and Right: Schematic views depicting the TraB symmetry alteration from side and top, respectively. Color-coding is as shown at left. Densities (gray) are extracted from the C1 reconstruction, which shows 17 strong densities (blue sites), 7 empty densities (magenta sites), and 2 weak densities (* at sites #12 and 16, magenta). Numbers refer to the 26 TraKNT domains and 17 connections to the TraBβ-barrel domains. c Left: TraV monomer (Rainbow color) displays an intraprotein symmetry alteration. A subset of the 26 TraVNT (residues C18-K55) bind 17 available sites in the CC. The flexible loop (residues K55-V150) bridges the symmetry mismatch, but can be deleted without affecting T4SSF function. Twenty-six copies of the TraVCT domain (residues V150-N204) comprise the ORC belt. Residues R90-G101 in the flexible loop associated with TraV S2 bind the 13 copies of TraKCT-S2 domains. Right: Schematic view depicting the TraV intraprotein symmetry alteration viewed from top. Densities associated with the traceable R90-G101 segment from TraV S2 are shown. The 4 magenta-colored TraVNT domains (randomly selected) have no traceable paths to TraVCT domains. The 13 TraVNT S2 domains (blue) are connected via the flexible loop to the TraVCT-S2 domains (rainbow). TraBβ-barrels comprise the CC (gray, inner ring). TraVNT-S1 domains connected to the TraVCT-S1 domains (gray, outer ring) have not been identified.