Abstract
Background: This study aimed to construct a clinical prediction model for osteosarcoma patients to evaluate the influence factors for the occurrence of lymph node metastasis (LNM).
Methods: In our retrospective study, a total of 1,256 patients diagnosed with chondrosarcoma were enrolled from the SEER (Surveillance, Epidemiology, and End Results) database (training cohort, n = 1,144) and multicenter dataset (validation cohort, n = 112). Both the univariate and multivariable logistic regression analysis were performed to identify the potential risk factors of LNM in osteosarcoma patients. According to the results of multivariable logistic regression analysis, A nomogram were established and the predictive ability was assessed by calibration plots, receiver operating characteristics (ROCs) curve, and decision curve analysis (DCA). Moreover, Kaplan-Meier plot of overall survival (OS) was plot and a web calculator visualized the nomogram.
Results: Five independent risk factors [chemotherapy, surgery, lung metastases, lymphatic metastases (M-stage) and tumor size (T-stage)] were identified by multivariable logistic regression analysis. What's more, calibration plots displayed great power both in training and validation group. DCA presented great clinical utility. ROCs curve provided the predictive ability in the training cohort (AUC = 0.805) and the validation cohort (AUC = 0.808). Moreover, patients in LNN group had significantly better survival than that in LNP group both in training and validation group.
Conclusion: In this study, we constructed and developed a nomogram with risk factors, which performed well in predicting risk factors of LNM in osteosarcoma patients. It may give a guide for surgeons and oncologists to optimize individual treatment and make a better clinical decision.
Keywords: nomogram, SEER, osteosarcoma, lymph node metastases, multicenter
Introduction
Osteosarcoma is a common malignant bone tumor. The primary treatment consisting of neoadjuvant therapy, surgery and postoperative chemotherapy have resulted in the 5-year overall survival rate of ~60% (1, 2). However, even with the treatment of surgery and chemotherapy, the prognosis for patients with metastatic osteosarcoma remains dismal (3, 4).The lung metastases, the primary target of metastasis in osteosarcoma, has five-year survival rates of ~30% (5, 6). In extrapulmonary metastatic osteosarcoma, patients with lymph node metastases (LNM) have worse clinical outcomes, with five-year survival rates of 10% (7). However, only 3% of patients with osteosarcoma are diagnosed with LNM, leading to the lack of adequate clinical data for exploring osteosarcoma LNM (8).Therefore, a population-based study to assess the LNM in osteosarcoma is imminent.
Furthermore, disease forecasting is a vital part of the medical research (9–18). As a visual prediction tool, nomogram lists each variable separately and assigns a corresponding score for each status (19). Based on these considerations, we mined the Surveillance, Epidemiology, and End Results (SEER) database to construct the nomogram and used data from four academic hospitals for independent validation. This study contributes to providing more personalized guidance for patient care and improving patients' prognosis.
Materials and Methods
Data Collection
In the present study, patients diagnosed with osteosarcoma between 2010 and 2016 were collected. The training group were extracted from the Surveillance, Epidemiology, and End Results (SEER) database with the SEER * Stat software version 8.3.6. And the third edition of the International Taxonomy of Oncology (ICDO-3), morphological code (9220) was used to identify osteosarcoma. The exclusion criteria of the training group were as follows: (1) patients with no positive pathology; (2) patients with unknown lymph node status and survival time; (3) more than one primary tumor.
Data of the validation group were obtained from four academic institutions, the Second Affiliated Hospital of Jilin University, the Second Affiliated Hospital of Dalian Medical University, the Liuzhou People's Hospital affiliated to Guangxi Medical University, and the Xianyang Central Hospital. And during the period of investigation, each center was responsible for the acquisition of data by three investigators. Two investigators were responsible for data extraction and the accuracy check was conducted by the third investigator. The exclusion criteria were consistent with the training group. For multicenter data, the study was approved by the ethics review committee of four medical institutions in China, the Second Affiliated Hospital of Jilin University, the Second Affiliated Hospital of Dalian Medical University, Liuzhou People's Hospital, and Xianyang Central Hospital (No. 2021-00-22) and was conducted in accordance with the guidelines of the Helsinki Declaration.
Demographic and clinical variables, including race, age, survival time, sex, primary site, grade, laterality, tumor size (T-stage), distance metastases (M-stage), surgery, radiation, chemotherapy, bone metastases and lung metastases were considered in this study.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation (SD), and categorical variables were expressed as frequency (proportions). The Student's t-test, Chi-square tests and Mann–Whitney tests were applied to continuous variables and categorical variables respectively with IBM SPSS Statistics version 26.0 (SPSS Inc., Chicago, Illinois, USA). Risk factors of the osteosarcoma were assessed using logistic regression. All analyzes were performed using R software version 3.6.2 (http://www.r-project.org/) including multiple R packages (Including regplot, rms, rmda and pROC). P values < 0.05 were considered statistically significant, and confidence intervals (CIs) were expressed as 95% confidence levels.
Construction, Validation and Clinical Utility of a Nomogram
The following variables were included in the univariate logistic regression analysis: race, age, survival time, sex, primary site, grade, laterality, tumor size, lymphatic metastasis, surgery, radiation, chemotherapy, bone metastases and lung metastases. According to the result of the univariate logistic regression analysis with the P value < 0.05, we performed the multivariable logistic regression analysis. And the Nomogram was constructed based on the results of multivariable logistic regression analysis with the P value < 0.05. Calibration plot and receiver operating characteristic (ROC) curves were used to evaluate the prediction performance of the nomogram. The higher the area under the curves (AUC) of ROC indicated the better model performance. In addition, the decision curve analysis (DCA) was used to evaluate the clinical utility of nomogram in decision-making. Based on the established nomogram, an interactive convenient web calculator was provided (https://drliwenle.shinyapps.io/LMOOapp/).
Results
Demographic Baseline Characteristics
As shown in Table 1, a total of 1,256 patients were enrolled. There was no statistically significant difference between the training group (n = 1,144) and validation group (n = 112) except the Race (P < 0.001) and Chemotherapy (P = 0.017).
Table 1.
Baseline data table of the training group and the validation group.
| Variable | level |
Overall (N = 1,256) |
SEER data (Training group, N = 1,144) | Multicenter data (validation group, N = 112) | p |
|---|---|---|---|---|---|
| Race (%) | Black | 168 (13.4) | 168 (14.7) | 0 (0.0) | <0.001 |
| Other | 228 (18.2) | 116 (10.1) | 112 (100.0) | ||
| White | 860 (68.5) | 860 (75.2) | 0 (0.0) | ||
| Age [mean (SD)] | NA | 33.31 (24.31) | 33.47 (24.26) | 31.62 (24.88) | 0.443 |
| Times [mean (SD)] | NA | 29.93 (22.69) | 29.91 (22.54) | 30.10 (24.24) | 0.933 |
| Sex (%) | Female | 573 (45.6) | 521 (45.5) | 52 (46.4) | 0.936 |
| Male | 683 (54.4) | 623 (54.5) | 60 (53.6) | ||
| Primary.Site (%) | Axis bone | 336 (26.8) | 309 (27.0) | 27 (24.1) | 0.349 |
| Limb bone | 817 (65.0) | 738 (64.5) | 79 (70.5) | ||
| Other | 103 (8.2) | 97 (8.5) | 6 (5.4) | ||
| Grade (%) | Moderately differentiated | 41 (3.3) | 41 (3.6) | 0 (0.0) | 0.124 |
| Poorly differentiated | 302 (24.0) | 279 (24.4) | 23 (20.5) | ||
| Undifferentiated; anaplastic | 560 (44.6) | 511 (44.7) | 49 (43.8) | ||
| Unknown | 324 (25.8) | 287 (25.1) | 37 (33.0) | ||
| Well-differentiated | 29 (2.3) | 26 (2.3) | 3 (2.7) | ||
| Laterality (%) | Left | 537 (42.8) | 494 (43.2) | 43 (38.4) | 0.08 |
| Not a paired site | 173 (13.8) | 163 (14.2) | 10 (8.9) | ||
| Right | 546 (43.5) | 487 (42.6) | 59 (52.7) | ||
| T (%) | T1 | 426 (33.9) | 388 (33.9) | 38 (33.9) | 0.294 |
| T2 | 569 (45.3) | 523 (45.7) | 46 (41.1) | ||
| T3 | 42 (3.3) | 35 (3.1) | 7 (6.2) | ||
| TX | 219 (17.4) | 198 (17.3) | 21 (18.8) | ||
| M (%) | M0 | 976 (77.7) | 892 (78.0) | 84 (75.0) | 0.547 |
| M1 | 280 (22.3) | 252 (22.0) | 28 (25.0) | ||
| Surgery (%) | No | 254 (20.2) | 230 (20.1) | 24 (21.4) | 0.834 |
| Yes | 1002 (79.8) | 914 (79.9) | 88 (78.6) | ||
| Radiation (%) | No | 1103 (87.8) | 999 (87.3) | 104 (92.9) | 0.119 |
| Yes | 153 (12.2) | 145 (12.7) | 8 (7.1) | ||
| Chemotherapy (%) | No | 274 (21.8) | 260 (22.7) | 14 (12.5) | 0.017 |
| Yes | 982 (78.2) | 884 (77.3) | 98 (87.5) | ||
| Bone metastases (%) | No | 1146 (91.2) | 1044 (91.3) | 102 (91.1) | 0.981 |
| Unknown | 52 (4.1) | 47 (4.1) | 5 (4.5) | ||
| Yes | 58 (4.6) | 53 (4.6) | 5 (4.5) | ||
| Lung metastases (%) | No | 988 (78.7) | 901 (78.8) | 87 (77.7) | 0.931 |
| Unknown | 48 (3.8) | 44 (3.8) | 4 (3.6) | ||
| Yes | 220 (17.5) | 199 (17.4) | 21 (18.8) |
Additionally, these patients were divided into two subgroups according to the LNM in Table 2. There were no significant differences in race, sex, laterality and radiation between the lymph node negative group (LNN, n = 1,104) and the lymph node positive (or unable to evaluate) group (LNP, n = 152). However, the other characteristics showed significant differences between the two groups.
Table 2.
Patient baseline table of lymphatic metastases.
| Level | Overall (N = 1,256) | No (N = 1,104) | Yes/Unable to evaluate (N = 152) | p | |
|---|---|---|---|---|---|
| Category (%) | Multicenter data (validation group) | 112 (8.9) | 93 (8.4) | 19 (12.5) | 0.133 |
| SEER data (Training group) | 1144 (91.1) | 1011 (91.6) | 133 (87.5) | ||
| Times [mean (SD)] | NA | 29.93 (22.69) | 30.95 (22.70) | 22.45 (21.18) | <0.001 |
| Race (%) | black | 168 (13.4) | 145 (13.1) | 23 (15.1) | 0.417 |
| Other | 228 (18.2) | 196 (17.8) | 32 (21.1) | ||
| White | 860 (68.5) | 763 (69.1) | 97 (63.8) | ||
| Age [mean (SD)] | NA | 33.31 (24.31) | 32.48 (23.79) | 39.35 (27.16) | 0.001 |
| Sex (%) | Female | 573 (45.6) | 498 (45.1) | 75 (49.3) | 0.37 |
| Male | 683 (54.4) | 606 (54.9) | 77 (50.7) | ||
| Primary.Site (%) | Axis bone | 336 (26.8) | 282 (25.5) | 54 (35.5) | 0.027 |
| Limb bone | 817 (65.0) | 732 (66.3) | 85 (55.9) | ||
| Other | 103 (8.2) | 90 (8.2) | 13 (8.6) | ||
| Grade (%) | Moderately differentiated | 41 (3.3) | 39 (3.5) | 2 (1.3) | <0.001 |
| Poorly differentiated | 302 (24.0) | 273 (24.7) | 29 (19.1) | ||
| Undifferentiated; anaplastic | 560 (44.6) | 508 (46.0) | 52 (34.2) | ||
| Unknown | 324 (25.8) | 257 (23.3) | 67 (44.1) | ||
| Well-differentiated | 29 (2.3) | 27 (2.4) | 2 (1.3) | ||
| Laterality (%) | Left | 537 (42.8) | 483 (43.8) | 54 (35.5) | 0.104 |
| Not a paired site | 173 (13.8) | 146 (13.2) | 27 (17.8) | ||
| Right | 546 (43.5) | 475 (43.0) | 71 (46.7) | ||
| T (%) | T1 | 426 (33.9) | 400 (36.2) | 26 (17.1) | <0.001 |
| T2 | 569 (45.3) | 526 (47.6) | 43 (28.3) | ||
| T3 | 42 (3.3) | 36 (3.3) | 6 (3.9) | ||
| TX | 219 (17.4) | 142 (12.9) | 77 (50.7) | ||
| M (%) | M0 | 976 (77.7) | 883 (80.0) | 93 (61.2) | <0.001 |
| M1 | 280 (22.3) | 221 (20.0) | 59 (38.8) | ||
| Surgery (%) | No | 254 (20.2) | 179 (16.2) | 75 (49.3) | <0.001 |
| Yes | 1002 (79.8) | 925 (83.8) | 77 (50.7) | ||
| Radiation (%) | No | 1103 (87.8) | 975 (88.3) | 128 (84.2) | 0.187 |
| Yes | 153 (12.2) | 129 (11.7) | 24 (15.8) | ||
| Chemotherapy (%) | No | 274 (21.8) | 208 (18.8) | 66 (43.4) | <0.001 |
| Yes | 982 (78.2) | 896 (81.2) | 86 (56.6) | ||
| Bone metastases (%) | No | 1146 (91.2) | 1046 (94.7) | 100 (65.8) | <0.001 |
| Unknown | 52 (4.1) | 14 (1.3) | 38 (25.0) | ||
| Yes | 58 (4.6) | 44 (4.0) | 14 (9.2) |
Univariate and Multivariable Logistic Regression Results
According to the univariate logistics regression analysis, we identified 10 prognostic factors including age, survival time, primary site, laterality, tumor size, lymph metastasis, surgery, chemotherapy, bone metastases and lung metastases in the training set (P < 0.05) (Table 3). Then, based on the above result, applying the multivariable logistics regression analysis, we figured out five independent prognostic factors including T-stage [TX: odds ratio (OR) 3.602,95%CI 1.710–5.483, P < 0.001], M-stage (M1: OR = 2.890, 1.463–5.709, P < 0.01), surgery (Yes: OR = 0.418, 0.247–0.706, P < 0.01), Chemotherapy (Yes: OR = 0.475, 0.267–0.819, P < 0.01)and Lung metastases (Unknown: OR = 9.407, 1.955–45.261, P < 0.01) (Table 3).
Table 3.
Univariate and multifactorial logistic regression analysis of risk factors for Lymph node metastases in patients with osteosarcoma.
| Variables | Univariate OR (95% CI) | p value | Multivariate OR (95% CI) | p value |
|---|---|---|---|---|
| Age (years) | 1.011 (1.004–1.018) | <0.01 | 0.993 (0.982–1.004) | 0.397 |
| Survival time (month) | 0.983 (0.974–0.992) | <0.001 | 0.998 (0.987–1.010) | 0.788 |
| Race | ||||
| White | Reference | Ref | Ref | Ref |
| Black | 1.248 (0.766–3.033) | 0.374 | / | / |
| Other | 0.993 (0.537–1.835) | 0.982 | / | / |
| Sex | ||||
| Male | Ref | Ref | Ref | Ref |
| Female | 1.163 (0.810–1.671) | 0.412 | / | / |
| Primary site | ||||
| Limb bones | Ref | Ref | Ref | Ref |
| Axis of a bone | 1.675 (1.133–2.478) | <0.05 | 0.946 (0.517–1.731) | 0.857 |
| Other | 1.286 (0.671–2.466) | 0.449 | 1.840 (0.806–4.198) | 0.148 |
| Grade | ||||
| Well-differentiated | Ref | Ref | Ref | Ref |
| Moderately differentiated | 1.282 (0.110–14.893) | 0.843 | / | / |
| Poorly differentiated | 2.569 (0.334–19.741) | 0.364 | / | / |
| Undifferentiated; anaplastic | 2.473 (0.328–18.673) | 0.380 | / | / |
| Unknown | 6.332 (0.840–47.705) | 0.073 | / | / |
| Laterality | ||||
| Left | Ref | Ref | Ref | Ref |
| Right | 1.395 (0.930–2.091) | 0.108 | 1.477 (0.920–2.371) | 0.106 |
| Other | 1.848 (1.102–3.101) | <0.05 | 1.113 (0.536–2.310) | 0.774 |
| T | ||||
| T1 | Ref | Ref | Ref | Ref |
| T2 | 1.121 (0.657–1.912) | 0.675 | 0.960 (0.540–1.707) | 0.889 |
| T3 | 2.528 (0.900–7.101) | 0.078 | 1.473 (0.476–4.559) | 0.502 |
| TX | 7.933 (4.780–13.167) | <0.001 | 3.062 (1.710–5.483) | <0.001 |
| M | ||||
| M0 | Ref | Ref | Ref | Ref |
| M1 | 2.506 (1.710–3.673) | <0.001 | 2.890 (1.463–5.709) | <0.01 |
| Surgery | ||||
| No | Ref | Ref | Ref | Ref |
| Yes | 0.197 (0.135–0.287) | <0.001 | 0.418 (0.247–0.706) | <0.01 |
| Radiation | ||||
| No | Ref | Ref | Ref | Ref |
| Yes | 1.524 (0.936–2.481) | 0.091 | / | / |
| Chemotherapy | ||||
| No | Ref | Ref | Ref | Ref |
| Yes | 0.289 (0.199–0.421) | <0.001 | 0.475 (0.276–0.819) | <0.01 |
| Bone metastases | ||||
| No | Ref | Ref | Ref | Ref |
| Yes | 3.260 (1.652–6.436) | <0.01 | 1.257 (0.570–2.772) | 0.571 |
| Unknown | 32.490 (16.267–64.892) | <0.001 | 2.159 (0.483–9.659) | 0.314 |
| Lung metastases | ||||
| No | Ref | Ref | Ref | Ref |
| Yes | 2.938 (1.887–4.573) | <0.001 | 0.902 (0.449–1.812) | 0.771 |
| Unknown | 45.225 (21.360–95.754) | <0.001 | 9.407 (1.955–45.261) | <0.01 |
Construction and Validation of Nomogram for Chondrosarcoma Patients
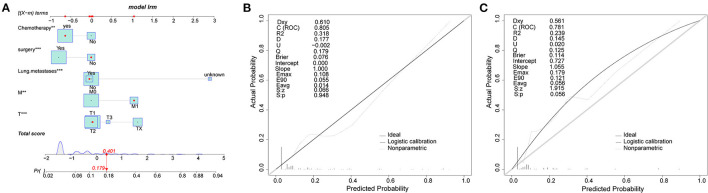
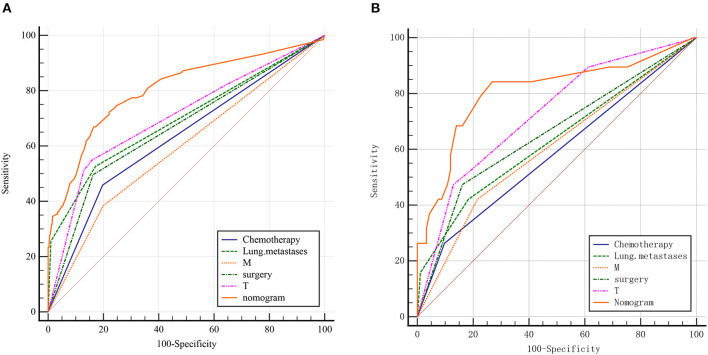
The nomogram contained five risk factors confirmed to be statistically significant by logistic regression analysis, including T-stage, M-stage, surgery, chemotherapy and lung metastases (Figure 1A). Calibration chart of nomogram showed a good consistency in the training and validation groups (Figures 1B,C). The AUC values of nomogram were 0.805 (95% CI 0.781–0.827) and 0.808 (95% CI 0.723–0.877) in the training group and the validation group respectively (Figures 2A,B). Furthermore, the ROC curve demonstrated a superior performance of the nomogram compared to the single variable, including chemotherapy (AUC = 0.631, 95%CI 0.602 to 0.659), lung metastases (0.697, 0.669 to 0.723), M-stage (AUC = 0.592, 95%CI 0.563 to 0.621), surgery (AUC = 0.667, 95%CI 0.639 to 0.694) and T-stage (0.706, 95%CI 0.678 to 0.732). The statistical results of validation group were consistent with the training group as shown in Table 4. In addition, an online web calculator was designed (https://drliwenle.shinyapps.io/LMOOapp/).
Figure 1.
(A) Nomogram for osteosarcoma patients. (B,C) are training cohorts and the validation cohorts calibration diagram respectively, which indicate good consistency.
Figure 2.
ROC curves for the training and validation group. (A) training group; (B) validation group.
Table 4.
AUC of training group and validation group.
|
SEER data (Training group) |
Multicenter data (validation group) |
|||||
|---|---|---|---|---|---|---|
| Variable | AUC | SE | 95% CI | AUC | SE | 95% CI |
| Chemotherapy | 0.631 | 0.0226 | 0.602 to 0.659 | 0.583 | 0.0541 | 0.486 to 0.676 |
| Lung metastases | 0.697 | 0.0239 | 0.669 to 0.723 | 0.631 | 0.0644 | 0.535 to 0.721 |
| M | 0.592 | 0.0221 | 0.563 to 0.621 | 0.603 | 0.062 | 0.506 to 0.694 |
| Surgery | 0.667 | 0.0225 | 0.639 to 0.694 | 0.656 | 0.0619 | 0.561 to 0.743 |
| T | 0.706 | 0.0256 | 0.678 to 0.732 | 0.728 | 0.0614 | 0.635 to 0.807 |
| Nomogram | 0.805 | 0.0231 | 0.781 to 0.827 | 0.808 | 0.065 | 0.723 to 0.877 |
Clinical Applicability of the Nomogram
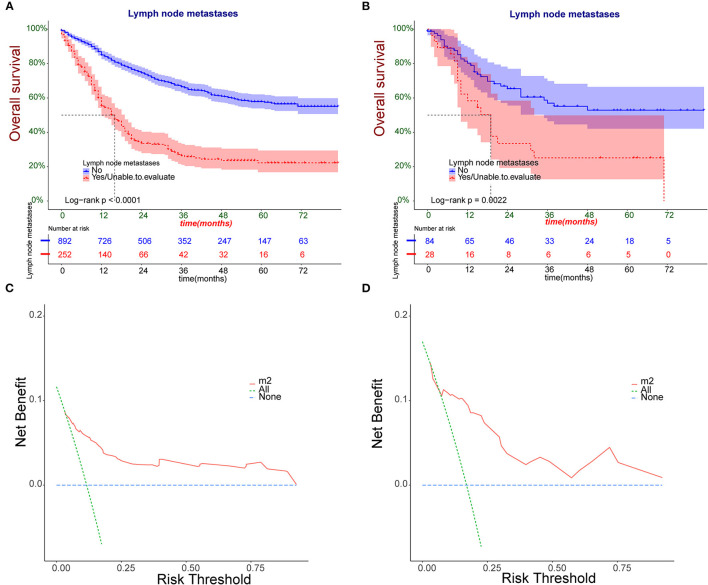
The Kaplan-Meier survival curves of training group were plotted (Figure 3A). The results revealed that the overall survival (OS) significantly decreased in patients with LNP comparing with the LNN (P < 0.001). Moreover, the threshold about 0.1 to 0.9 had the maximum benefit range of the model as shown in the DCA curve (Figures 3C,D). The Kaplan-Meier survival curves of validation group displayed the same trend between the two groups (P <0.001) (Figure 3B).
Figure 3.
(A,B) The Kaplan-Meier survival analysis patients with osteosarcoma in training and validation group. (C,D) Nomogram decision curve (DCA) of the training and validation group.
Discussion
Osteosarcoma metastases, which are typically secondary to hematogenous dissemination and the occurrence of lymph system is extremely rare, have been identified to be significantly associated with poor prognosis (1–4, 7, 20, 21). Comparing to the most common lung metastases, osteosarcoma patients with lymph node involvement have a worse prognosis, suggesting that the invasion of the lymph nodes is an important indicator for the assessment of malignancy stage and the selection of a correct treatment protocol (22, 23).Meanwhile, studies points out that FDG-PET and 99mTc-labeled biomineralization nanoprobe are effective in early diagnosis of metastatic lymph nodes in osteosarcoma (24–27). Therefore, real-time lymph node surveillance and radical treatment for osteosarcoma patients with a high risk of lymph node metastasis will improve patient survival (8). In this study, we identified five independent risk factors associated with LNM and provided a convenient nomogram prediction model and a web calculator on the basis of the model.
Surgery and chemotherapy are the most reliable and effective treatment options for prolonging the lives of patients and have been followed in several clinical trials (28–30). According to the multivariable logistic analysis, chemotherapy and surgery were crucial prognostic indicators. Surgery and chemotherapy contributed to improve patient prognosis, which were consistent with the result of the KM survival curve. This suggests that timely and effective treatment plays an important role in controlling lymph node metastasis and enhancing OS (15, 17, 31, 32). Unfortunately, the relationship between radiotherapy and lymph node metastasis has not been proved. A possible reason for these results would be the uncertainty of radiation therapy in cancer treatment along with the development of radiation-associated osteosarcoma make the safety of chemotherapy need to be further ensured (33, 34).
Due to the lack of lymphatic drainage in normal cortical bone and spongy bone, LNM is rare in bone sarcomas (35, 36).Regional lymph node involvement in osteosarcoma may be owing to the infiltration of the enlarged tumor parenchyma into the periphery, such as the joint capsule or synovium, leading to dissemination into the lymphatic system (37, 38).Our study reported T-stage as a significant predictor of LNM, which was also consistent with previous findings (7).Meanwhile, because of the fact that the peak incidence of the osteosarcoma is 15–19 years of age, more than 80% of patients achieve limb salvage through surgery (4, 28, 38).In this respect, the surgeons need pay more attention to the status of the regional lymph nodes during the resection of larger osteosarcoma in order to eradicate the sarcoma and preserve the limb. M-stage indicates distant metastasis in osteosarcoma, and it is also a risk factor associated with LNM. In the study by Thampi et al. osteosarcoma lymphatic metastasis was significantly associated with distant metastasis (7).Furthermore, since the lung was the most important target organ for distant metastasis in osteosarcoma, we separated this variable from distant metastasis to emphasize the significant role played by lung metastasis in assessing lymph node status. Patients with lung metastases express the characteristic biomarker, such as KEAP, Matrix-Gla and Rab22a (39–41). Considering the intense correlation we found between lung metastasis and lymph node involvement, these reported biomarkers would be triggers for LNM. Therefore, this provides inspiration to further molecular biology studies focusing on lymph node metastasis in osteosarcoma.
We constructed a novel nomogram to assess the risk of developing lymph node metastasis in osteosarcoma, and the discriminatory of any individual predictor was inferior to that of nomogram, suggesting that the nomogram model indicated promising prospects for tumor surveillance and clinical decision making. Although some predictive nomograms have been reported in previous studies, our study complements previous work. Compared to Dong et al.'s study, external independent validation consisting of multiple academic centers is a prominent feature of this study, and the inclusion of multiple ethnic groups enhances the credibility of the results (8). Moreover, we provide a convenient and digital prediction tool for users. By analyzing the clinical characteristics and associated risk factors, we improve the prediction of lymph node metastasis risk in osteosarcoma and provide a basis for individualized treatment and follow-up strategies. The web-based calculator constructed in this study is an easy-to-use clinical tool that helps to promote personalized treatment.
Finally, one obvious limitation in this study was that the statistically significant difference between the training group and validation groups in chemotherapy may have an influence on the results. Another limitation was the lack of a more thoroughly analysis due to the inadequacy of systematic and prospective data.
Conclusion
In conclusion, we constructed a novel nomogram model to predict risk factors for osteosarcoma patients developing LNM, including T-stage, M-stage, surgery, chemotherapy and lung metastases based on epidemiological characteristics obtained from the SEER database and the multicenter hospitals. By combining DCA curve, ROC curve, KM curve, web calculator and external validation, our nomogram provided an accurate assessment for individualized risk of lymph node metastasis which was helpful for clinicians to make better surgery decisions.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics Statement
The SEER database is a comprehensive data source developed based on population data and updated annually since its launch in 1973. It is public and identifiably accessible that data analysis is treated as non-human subjects by the Office for Human Research Protections. As such, no institutional review board approval and informed consent were required. For multicenter data, the study was approved by the ethics review committee of four medical institutions in China, the Second Affiliated Hospital of Jilin University, the Second Affiliated Hospital of Dalian Medical University, 291 Liuzhou People's Hospital, and Xianyang Central Hospital (No. 2021-00-22) and was conducted in accordance with the guidelines of the Helsinki.
Author Contributions
CY, RW, QL, and XL designed the study. WeL and SD performed the study, analyzed the data, and wrote the manuscript. BW and HW provided the expert consultations and clinical suggestions. CX and KZ conceived of the study, participated in its design and coordination. WaL and ZH helped to draft the manuscript. All authors reviewed the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank China's multicenter for providing us individual patient data. The multicenter data were obtained from four medical institutions in China, the Second Affiliated Hospital of Jilin University, the Second Affiliated Hospital of Dalian Medical University, Liuzhou People's Hospital, and Xianyang Central Hospital.
Glossary
Abbreviations
- SEER
Surveillance, Epidemiology, and End Results database
- ROC
receiver operating characteristic
- DCA
decision curve analysis
- CI
confidence interval
- AUC
area under the curve
- LNN
lymph node negative
- LNM
lymph node metastases
- ICD
International Classification of Diseases
- SD
standard deviation
- KM curves
Kaplan-Meier curves
- OR
odds ratio.
References
- 1.Marina N, Smeland S, Bielack S, Bernstein M, Jovic G, Hook J, et al. “MAPIE vs MAP as postoperative chemotherapy in patients with a poor response to preoperative chemotherapy for newly-diagnosed osteosarcoma: results from EURAMOS-1 (Paper 032),” in Presented at Connective Tissue Oncology Society (2014). [Google Scholar]
- 2.Tian Z, Niu X, Yao W. Receptor tyrosine kinases in osteosarcoma treatment: which is the key target? Front Oncol. (2020) 10:1642. 10.3389/fonc.2020.01642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kager L, Zoubek A, Pötschger U, Kastner U, Flege S, Kempf-Bielack B, et al. Primary metastatic osteosarcoma: presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol. (2003) 21:2011–8. 10.1200/JCO.2003.08.132 [DOI] [PubMed] [Google Scholar]
- 4.Hattori H, Yamamoto K. Lymph node metastasis of osteosarcoma. J Clin Oncol. (2012) 30:345–9. 10.1200/JCO.2012.42.3384 [DOI] [PubMed] [Google Scholar]
- 5.Kempf-Bielack B. Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS). J Clin Oncol. (2005) 23:559–68. 10.1200/JCO.2005.04.063 [DOI] [PubMed] [Google Scholar]
- 6.Zheng B, Zhou C, Qu G, Ren C, Yue B. VEGFR2 promotes metastasis and PD-L2 expression of human osteosarcoma cells by activating the STAT3 and RhoA-ROCK-LIMK2 pathways. Front Oncol. (2020) 10:543562. 10.3389/fonc.2020.543562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thampi S, Matthay KK, Goldsby R, Dubois SG. Adverse impact of regional lymph node involvement in osteosarcoma. Eur J Cancer. (2013) 49:3471–6. 10.1016/j.ejca.2013.06.023 [DOI] [PubMed] [Google Scholar]
- 8.Dong Y, Wu W, Kang H, Xiong W, Li F. Risk factors of regional lymph node (RLN) metastasis among patients with bone sarcoma and survival of patients with RLN-positive bone sarcoma. Ann Transl Med. (2021) 9:48. 10.21037/atm-20-4681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar R, Tripathi R, Marchang N, Srivastava G, Gadekallu TR, Xiong NN. A secured distributed detection system based on IPFS and blockchain for industrial image and video data security. J Parallel Distributed Comput. (2021) 152:128–43. 10.1016/j.jpdc.2021.02.022 [DOI] [Google Scholar]
- 10.Agrawal S, Sarkar S, Srivastava G, Maddikunta P, Gadekallu TR. Genetically optimized prediction of remaining useful life. Sustain Comput. (2021) 31:100565. 10.1016/j.suscom.2021.10056534695985 [DOI] [Google Scholar]
- 11.Naeem A, Javed AR, Rizwan M, Abbas S, Gadekallu TR. DARE-SEP: a hybrid approach of distance aware residual energy-efficient SEP for WSN. IEEE Trans Green Commun Netw. (2021) 5:611–21. 10.1109/TGCN.2021.306788527295638 [DOI] [Google Scholar]
- 12.Gadekallu TR, Alazab M, Kaluri R, Maddikunta P, Parimala M. Hand gesture classification using a novel CNN-crow search algorithm. Complex Intell Syst. (2021) 7:1855–68. 10.1007/s40747-021-00324-x26394431 [DOI] [Google Scholar]
- 13.Gadekallu TR, Manoj MK, Sivarama KS, Kumar N, Bhattacharya S. Blockchain based attack detection on machine learning algorithms for IoT based E-health applications. IEEE Internet Things Mag. (2020) 4:30–33. 10.1109/IOTM.1021.200016027295638 [DOI] [Google Scholar]
- 14.Tang Z, Zhu R, Lin P, He J, Wang H, Huang Q, et al. A hardware friendly unsupervised memristive neural network with weight sharing mechanism. Neurocomputing. (2019) 332:193–202. 10.1016/j.neucom.2018.12.049 [DOI] [Google Scholar]
- 15.Wu EQ, Hu D, Deng PY, Tang Z, Ren H. Nonparametric bayesian prior inducing deep network for automatic detection of cognitive status. IEEE Trans Cybern. (2020) 51:5483–96. [DOI] [PubMed] [Google Scholar]
- 16.Tang Z, Chen Y, Ye S, Hu R, Chang S. Fully Memristive spiking-neuron learning framework and its applications on pattern recognition and edge detection. Neurocomputing. (2020) 403:80–7. 10.1016/j.neucom.2020.04.012 [DOI] [Google Scholar]
- 17.Wu EQ, Deng PY, Qu XY, Tang Z, Sheng RSF. Detecting fatigue status of pilots based on deep learning network using EEG signals. IEEE Trans Cogn Dev Syst. (2020) 13:575–85. 10.1109/TCDS.2019.296347627295638 [DOI] [Google Scholar]
- 18.Tang Z, Zhu R, Hu R, Chen Y, Chang S. A multilayer neural network merging image preprocessing and pattern recognition by integrating diffusion and drift memristors. IEEE Trans Cogn Dev Syst. (2020) 13:645–56. 10.1109/TCDS.2020.300337727295638 [DOI] [Google Scholar]
- 19.Balachandran VP, Gonen M, Smith JJ, Dematteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. (2015) 16:e173–e80. 10.1016/S1470-2045(14)71116-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meazza C, Scanagatta P. Metastatic osteosarcoma: a challenging multidisciplinary treatment. Expert Rev Anticancer Ther. (2016) 16:543–56. 10.1586/14737140.2016.1168697 [DOI] [PubMed] [Google Scholar]
- 21.Wang T, Quan Y, Shen XS, Gadekallu TR, Wang W, Dev K, et al. Privacy-enhanced retrieval technology for the cloud-assisted internet of things. IEEE Trans Ind Inform. (2021) 14:1551–3203. 10.1109/TII.2021.310354727295638 [DOI] [Google Scholar]
- 22.Alitalo K. The lymphatic vasculature in disease. Nat Med. (2011) 17:1371–80. 10.1038/nm.2545 [DOI] [PubMed] [Google Scholar]
- 23.Wang W, Qiu C, Yin Z, Srivastava G, Gadekallu TR, Alsolami F, et al. Blockchain and PUF-based lightweight authentication protocol for wireless medical sensor networks. IEEE Internet Things J. (2021) 1–1. 10.1109/JIOT.2021.311776227295638 [DOI] [Google Scholar]
- 24.Xu Z, Wang Y, Han J, Xu Q, Ren J, Xu J, et al. Noninvasive multimodal imaging of osteosarcoma and lymph nodes using a 99mTc-labeled biomineralization nanoprobe. Anal Chem. (2018) 90:4529–34. 10.1021/acs.analchem.7b04925 [DOI] [PubMed] [Google Scholar]
- 25.Volker T, Denecke T, Steffen I, Misch D, Schonberger S, Plotkin M, et al. Positron emission tomography for staging of pediatric sarcoma patients: results of a prospective multicenter trial. J Clin Oncol. (2007) 25:5435. 10.1200/JCO.2007.12.2473 [DOI] [PubMed] [Google Scholar]
- 26.Wang W, Fida MH, Lian Z, Yin Z, Pham QV, Gadekallu TR, et al. Secure-enhanced federated learning for AI-empowered electric vehicle energy prediction. IEEE Consum Electron Mag. (2021). 10.1109/MCE.2021.311691727295638 [DOI] [Google Scholar]
- 27.Xiong H, Jin C, Alazab M, Yeh KH, Wang H, Gadekallu TRR, et al. On the design of blockchain-based ECDSA with fault-tolerant batch verication protocol for blockchain-enabled IoMT. IEEE J Biomed Health Inform. (2021). 10.1109/JBHI.2021.3112693 [DOI] [PubMed] [Google Scholar]
- 28.Ritter J, Bielack SS. Osteosarcoma. Ann Oncol. (2010) 21(Suppl 7):vii320–5. 10.1093/annonc/mdq276 [DOI] [PubMed] [Google Scholar]
- 29.Pramanik R, Agarwala S, Gupta YK, Thulkar S, Bakhshi S. Metronomic chemotherapy vs best supportive care in progressive pediatric solid malignant tumors: a randomized clinical trial. Jama Oncol. (2017) 3:1222–7. 10.1001/jamaoncol.2017.0324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piperno-Neumann S, Le Deley MC, Rédini F, Pacquement H, Marec-Bérard P, Petit P, et al. Zoledronate in combination with chemotherapy and surgery to treat osteosarcoma (OS2006): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. (2016) 17:1070–80. 10.1016/S1470-2045(16)30096-1 [DOI] [PubMed] [Google Scholar]
- 31.Huang Z, Hu C, Liu K, Yuan L, Hu C. Risk factors, prognostic factors, and nomograms for bone metastasis in patients with newly diagnosed infiltrating duct carcinoma of the breast: a population-based study. BMC Cancer. (2020) 20:1145. 10.1186/s12885-020-07635-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu EQ, Zhou MC, Hu D, Zhu L, Ren H. Self-paced dynamic infinite mixture model for fatigue evaluation of pilots' brains. IEEE Trans Cybern. (2020) 2168–267. 10.1109/TCYB.2020.3033005 [DOI] [PubMed] [Google Scholar]
- 33.Siontis BL, Mchugh JB, Roberts E, Zhao L, Chugh R. Differential outcomes and biologic markers of radiation-associated vs. sporadic osteosarcoma: a single-institution experience. Front Oncol. (2020) 9:1523. 10.3389/fonc.2019.01523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu EQ, Zhu LM, Li GJ, Li HJ, Zhou GR. Nonparametric hierarchical hidden semi-markov model for brain fatigue behavior detection of pilots during flight. IEEE Trans Intell Transport Syst. (2021) 1–12. 10.1109/TITS.2021.305280127295638 [DOI] [Google Scholar]
- 35.Cleary MX, Fayad LM, Ahlawat S. Popliteal lymph nodes in patients with osteosarcoma: are they metastatic? Skeletal Radiol. (2020) 49:1807–17. 10.1007/s00256-020-03498-6 [DOI] [PubMed] [Google Scholar]
- 36.Wu EQ, Lin CT, Zhu LM, Tang ZR, Jie YW, Zhou GR. Fatigue detection of pilots' brain through brains cognitive map and multilayer latent incremental learning model. IEEE Trans Cybern. (2021) 1–13. 10.1109/TCYB.2021.3068300 [DOI] [PubMed] [Google Scholar]
- 37.Dirik Y, Çinar A, Yumrukçal F, Eralp L. Popliteal lymph node metastasis of tibial osteoblastic osteosarcoma. Int J Surg Case Rep. (2014) 5:840–4. 10.1016/j.ijscr.2014.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. (2002) 20:776–90. 10.1200/JCO.2002.20.3.776 [DOI] [PubMed] [Google Scholar]
- 39.Xu H, Zhu X, Bao H, Shek TW, Huang Z, Wang Y, et al. Genetic and clonal dissection of osteosarcoma progression and lung metastasis. Int J Cancer. (2018) 143:1134−42. 10.1002/ijc.31389 [DOI] [PubMed] [Google Scholar]
- 40.Zandueta C, Ormazábal C, Perurena N, Martínez-Canarias S, Zalacaín M, Julián MS, et al. Matrix-Gla protein promotes osteosarcoma lung metastasis and associates with poor prognosis. J Pathol. (2016) 239:438–49. 10.1002/path.4740 [DOI] [PubMed] [Google Scholar]
- 41.Liao D, Zhong L, Yin J, Zeng C, Wang X, Huang X, et al. Chromosomal translocation-derived aberrant Rab22a drives metastasis of osteosarcoma. Nat Cell Biol. (2020) 22:868–81 10.1038/s41556-020-0522-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.