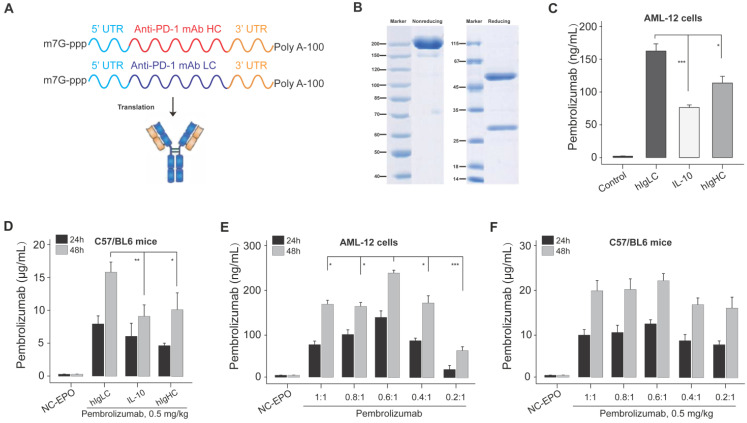
Figure 1.
Design and optimization of Pembrolizumab mRNA for LNP delivery. (A) Schematic of the structure of mRNA-encoded the heavy and light chains of the Pembrolizumab. (B) SDS-PAGE detection of the Pembrolizumab translated from mRNA in supernatants from Expi293F cells. (C) The expression levels of Pembrolizumab in supernatants of AML-12 cell transfection with mRNA-encoded Pembrolizumab with three different SPs for 24 h. (D) The serum levels of Pembrolizumab in the C57BL/6 received the single injection of 0.5 mg/kg LNP- Pembrolizumab mRNA with three different SPs. (E) The serum levels of Pembrolizumab in the C57BL/6 received the single injection of 0.5 mg/kg LNP- Pembrolizumab mRNA with different molar ratios. *p < 0.05, **p < 0.01, ***p < 0.001. One-way ANOVA with Tukey's post hoc test. Error bars show the SEM, n=10.