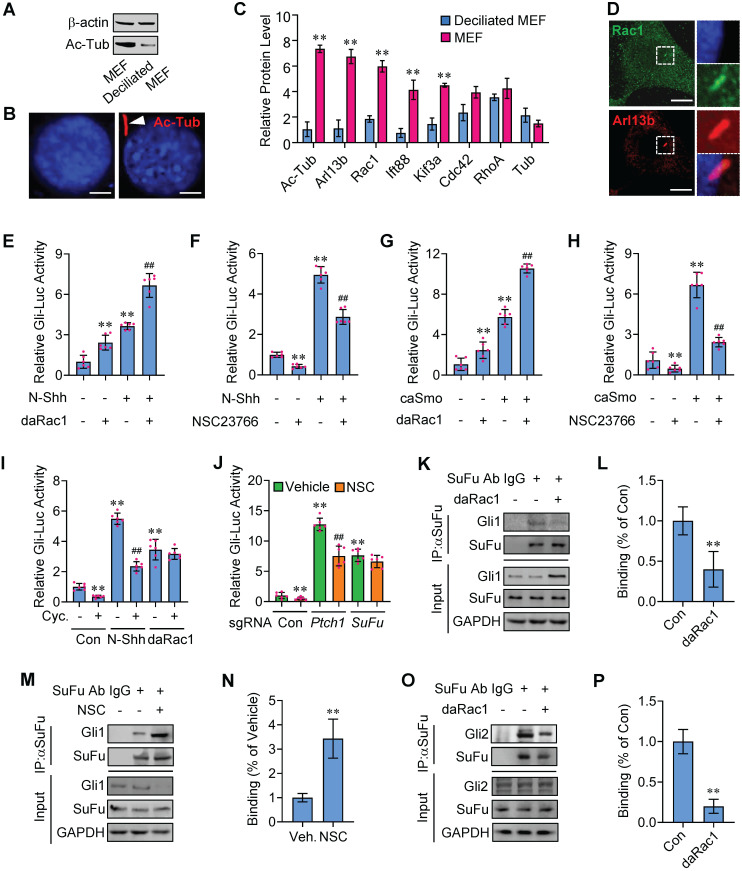
Figure 1.
Potentiation of Hh signaling by Rac1 activation. (A) Total cellular membrane lysates from MEFs in high densities and deciliated MEFs in low densities were subjected to immunoblotting. The membrane lysates of MEFs from high densities are enriched for the ciliary component acetylated tubulin (Ac-Tub). (B) Immunofluorescence staining (red) for Ac-Tub in MEFs and deciliated MEFs. Nuclei were counterstained by DAPI. Bar, 20 μm. (C) LC-MS/MS quantitation of cilia markers and Rho family of small GTPases extracted from deciliated MEFs and MEFs. N=3. (D) Immunofluorescence staining for Rac1 in MEF. Primary cilia were indicated by Arl13b staining. Nuclei were counterstained by DAPI. Bar, 15 μm. (E) C3H10T1/2 cells were transiently transfected with a Gli luciferase reporter together with daRac1 and cultured with or without N-Shh at 100 ng/ml for 24 h. Total cell lysates were subjected to luciferase assay. (F) C3H10T1/2 cells were transiently transfected with a Gli luciferase reporter and cultured with or without N-Shh at 100 ng/ml or NSC23766 at 10 μg/ml for 24 h. Total cell lysates were subjected to luciferase assay. (G) C3H10T1/2 cells were transiently transfected with a Gli luciferase reporter together with daRac1 and caSmo and cultured for 24 h. Total cell lysates were subjected to luciferase assay. (H) C3H10T1/2 cells were transiently transfected with a Gli luciferase reporter together with caSmo and cultured with or without NSC23766 at 10 μg/ml for 24 h. Total cell lysates were subjected to luciferase assay. (I) C3H10T1/2 cells were transiently transfected with a Gli luciferase reporter together with daRac1 and cultured with or without Cyclopamine (Cyc.) at 5 μM for 24 h. Total cell lysates were subjected to luciferase assay. (J) Ptch1-knockout (sgRNA-Ptch1), SuFu-knockout (sgRNA-SuFu) or control (sgRNA-Con) C3H10T1/2 cells were transiently transfected with a Gli luciferase reporter for 48 h and cultured with or without NSC23766 (NSC) at 10 μg/ml for 24 h. Total cell lysates were subjected to luciferase assay. (K) C3H10T1/2 cells were transfected with daRac1. Total cell lysates (Input) and anti-SuFu immunoprecipitates (IP, SuFu Ab, +) from total cell lysates were analyzed by immunoblotting with anti-SuFu and anti-Gli1 antibodies. IgG was used as a negative control for IP. (L) Quantification via densitometry (n=3) and statistical analysis of Gli1 bands of (K). (M) C3H10T1/2 cells were cultured with NSC23766 (NSC) at 10 μg/ml for 24 h. Total cell lysates (Input) and anti-SuFu immunoprecipitates (IP, SuFu Ab, +) from total cell lysates were analyzed by immunoblotting with anti-SuFu and anti-Gli1 antibodies. IgG was used as a negative control for IP. (N) Quantification via densitometry (n=3) and statistical analysis of Gli1 bands of (M). (O) C3H10T1/2 cells were transfected with or without daRac1. Total cell lysates (Input) and anti-SuFu immunoprecipitates (IP, SuFu Ab, +) from total cell lysates were analyzed by immunoblotting with anti-SuFu and anti-Gli2 antibodies. IgG was used as a negative control for IP. (P) Quantification via densitometry (n=3) and statistical analysis of Gli2 bands of (O). Protein abundance normalized to GAPDH, respectively. *p < 0.05; **, ##p < 0.01; n=6 in (E-J), error bar, SD.