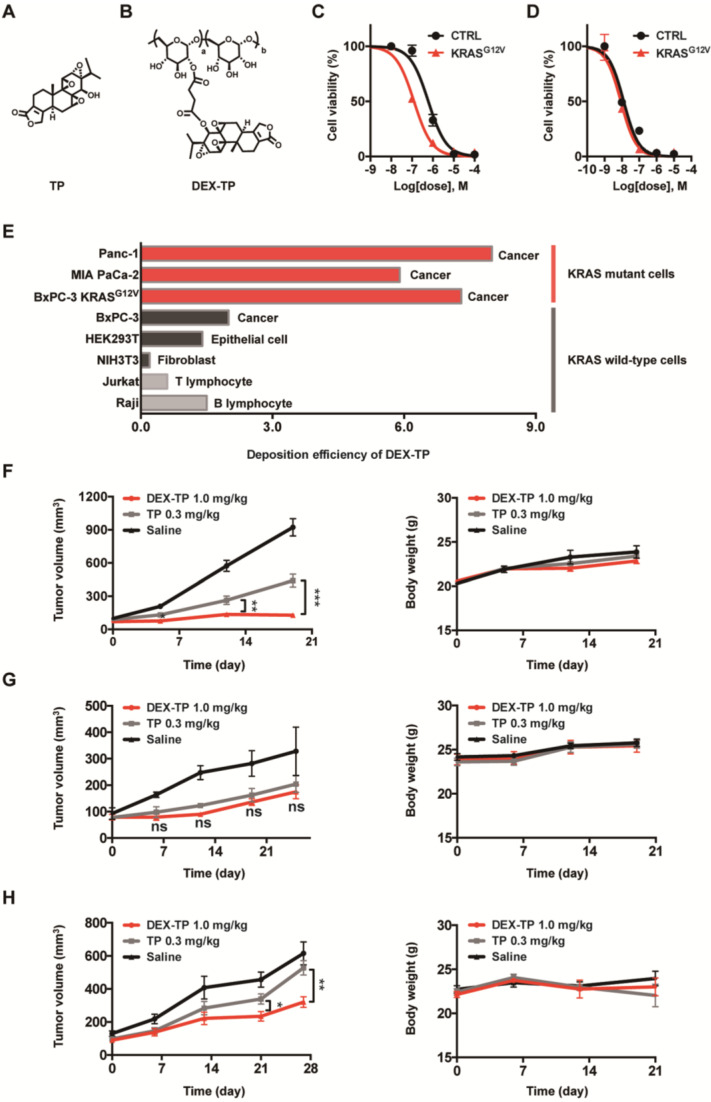
Figure 2.
Enhanced efficacy, cellular deposition efficiency, and tumor growth inhibition of DEX-TP towards KRAS mutant cancer. (A and B) Chemical structures of TP (A) and DEX-TP (B). (C and D) Drug efficacy of DEX-TP (C) and TP (D) towards BxPC-3 (CTRL) and BxPC-3 KRASG12V (KRASG12V) cells. Error bars indicate the mean and SD, n=3. (E) Cellular deposition efficiency of DEX-TP under different KRAS gene backgrounds. (F, G and H) Tumor growth curves (left) and body weight surveillance (right) under the following drug treatments: MIA PaCa-2-derived subcutaneous model (F), n (saline) = 7, n (TP) = 8, n (DEX-TP) = 8, and error bars indicate the mean and SD; BxPC-3 derived subcutaneous model (G), n (saline) = 4, n (TP) = 6, n (DEX-TP) = 6, and error bars indicate the mean and SD; BxPC-3 KRASG12V-derived subcutaneous mode (H), n (saline) = 7, n (TP) = 7, n (DEX-TP) = 7, and error bars indicate the mean and SD. All treatments were given every two days for a total of 7 treatments via tail vein injection, and doses are denoted as TP equivalents.