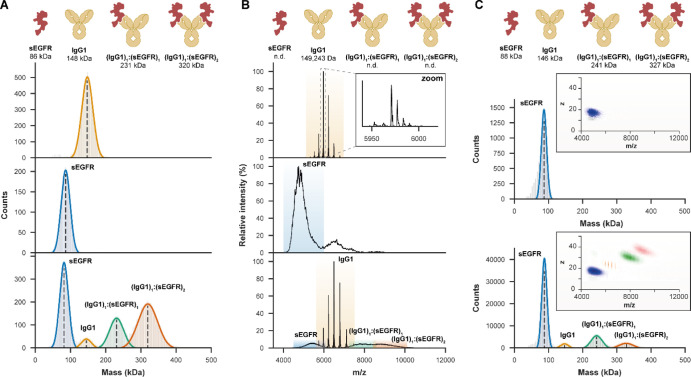
Figure 2.
MP and CD-MS may overcome certain limitations of native MS in the mass measurements of highly heterogeneous antibody–antigen complexes. (A) MP provides an average mass for IgG1 (upper panel) and sEGFR (middle) and is not hampered by the high micro-heterogeneity of the latter. When 2 μM IgG1 was incubated with 5 μM of sEGFR to form (IgG1)1:(sEGFR)1 and (IgG1)1:(sEGFR)2 complexes, jump dilution MP could resolve these highly heterogeneous species (lower). (B) Although native MS on samples at the same concentrations provided superior mass resolution and accuracy for free IgG1 (upper), resolving individual glycoforms (zoom), the high microheterogeneity of sEGFR, measured separately (middle) and in antibody–antigen complexes (lower), resulted in unresolved features. In these experiments, overlapping charge states prevented mass measurements of these species. (C) More accurate masses could be obtained by native CD-MS, measuring in two dimensions m/z and z (insets) for sEGFR (upper) and all co-occurring species involving IgG1 and sEGFR (lower). For these experiments, the same native MS samples were diluted 20-fold, leading to re-equilibration and thus a lower binding occupancy.