Figure 4.
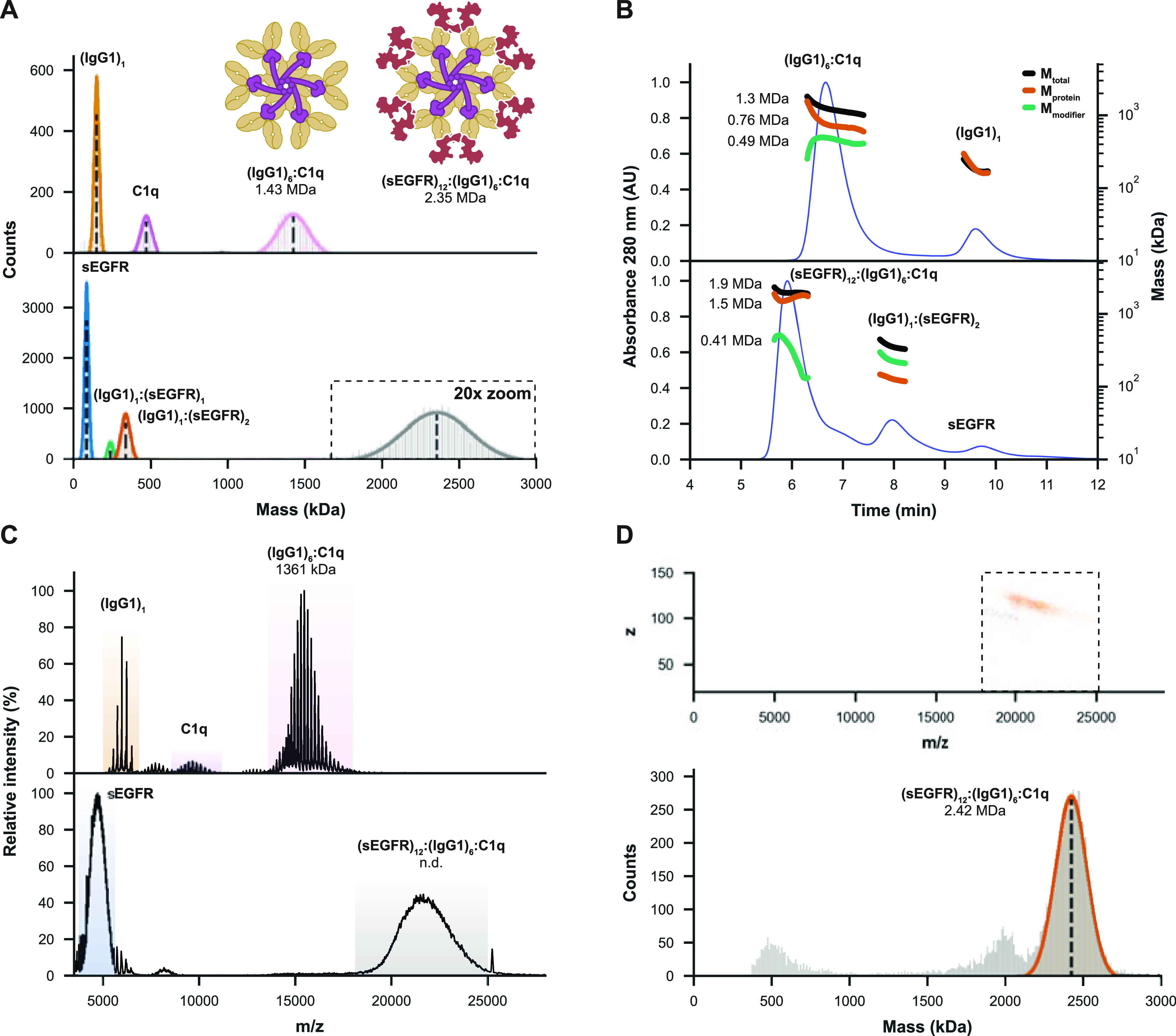
MP and CD-MS successfully determine the mass and stoichiometry of highly heterogeneous (sEGFR)12:(IgG1)6:C1q immune complexes. (A) MP measurements of IgG1-RGY incubated with C1q reveal the formation of (IgG1)6:(C1q)1 complexes, with nearly all IgG hexamers occupied. When incubating C1q with pre-formed (IgG1)6:(sEGFR)12, MP resolves a 2.35 MDa complex, likely corresponding to (sEGFR)12:(IgG1)6:(C1q)1. (B) SEC-MALS-UV-RI analysis similarly reveals the formation of ∼1.3 MDa (IgG1)6:(C1q)1 (with (IgG1)6 measured as the 0.76 MDa “protein” and C1q as a 0.49 MDa “modifier”). When sEGFR was added, SEC-MALS-UV-RI revealed the formation of larger complexes of around 1.9 MDa (1.5 MDa for (sEGFR)12:(IgG1)6 with a 0.41 MDa modifier). (C) Measurement of the same samples by native MS reveals an accurate mass for (IgG1)6:(C1q)1, but the technique struggles with complexes involving sEGFR. Larger ion species were detected in such experiments, but they could not be charge-resolved. (D) Single-particle measurements of the distribution around m/z 21,000 by CD-MS (top) revealed a mass of 2.42 MDa (bottom) corresponding to the expected mass of the full (sEGFR)12:(IgG1)6:(sEGFR)12 complex (bottom).
