Opinion statement
The increased risk for cardiovascular events in aging cancer survivors and those undergoing certain chemotherapeutic treatments has raised concern for more rigorous screening and surveillance methods above that of the general population. At this time, there are limited guidelines for how to best manage this vulnerable cohort. Questions regarding timing of screening, choice of imaging modality and risk reduction strategies—especially in those patients with known atherosclerotic disease—remain to be elucidated. Over a decade of case series, retrospective studies and clinical trials have shed light on the evolving role of cardiac computed tomography (CT) in this population, of which there is a relative paucity of data regarding its potential utility in the specific cardio-oncology population. Focusing on ability of cardiac CT to evaluate multiple cardiac and vascular structures, provide diagnostic and prognostic information, as well as assist interventional and surgical colleagues in surgical/percutaneous valve replacement and revascularization strategies is the premise for this review.
Keywords: Cardiotoxicity, Radiation-induced valvulopathy, Vasculotoxicity, Cardiac computed tomography angiography
Introduction
In 2018, an estimated 1,735,350 new cancer cases—with 609,640 cancer deaths—occurred in the United States [1]. In addition, an estimated 15.5 million cancer survivors were also residing in the USA in 2018, representing 4.8% of the total population; 62% of which were 65 years or older. This number is expected to grow to 26.1 million survivors by 2040 [2]. Given the improved survival in cancer patients over the last several decades due to significant advances in drug therapy and surveillance efforts, the need to address cardiovascular outcomes in this aging population during and after treatment has become a focus of interest in the emerging multi-disciplinary field of cardio-oncology.
Cancer patients who have been exposed to high-risk chemotherapeutic regimens and/or mediastinal radiation are pre-disposed to both short- and long-term cardiovascular complications [3, 4]. Moreover, recently, it has been reported that 1 in 10 patients undergoing percutaneous coronary interventions had either a current or historical diagnosis of cancer [5]. Years of prospective analyses from the Childhood Cancer Survivor Study have demonstrated increased prevalence of hypertension, coronary artery disease (CAD), heart failure, and valvular heart disease amongst > 10,000 adult survivors of childhood cancer [6]. In adults with colorectal cancer, cardiovascular mortality may surpass that of colorectal cancer death 8 years post-diagnosis [7, 8]. Likewise, cardiovascular morbidity and mortality are an important competing risk in elderly female breast cancer patients [9]. That being said, the natural timeline of developing cardiovascular disease in both childhood and adult cancer survivors remains unclear, calling for a need to refine risk stratification in this population. Furthermore, a recent analysis from the Multi-Ethnic Study of Atherosclerosis (MESA) suggested that coronary atherosclerosis and cancer may develop during the same phase of life, supported by the notion of increased risk of new coronary artery calcium development in the subjects who also developed cancer during follow-up [10]. The association of increased cardiovascular risk burden with higher cardiovascular mortality and death supports the notion for a better control of modifiable risk factors [11]. Importantly, better cardiovascular risk profiles are associated with lower healthcare cost and better outcomes in cancer patients [12].
Cardiac CT, which includes non-contrast CT and CT angiography (CTA), is already used as non-invasive imaging modality with superior visualization of coronary anatomy that can risk stratify, diagnose and guide treatment decisions in this at-risk population. Cardiac CT provides high-resolution imaging of coronary, pericardial, structural, and vascular anatomy, and offers potential non-invasive insights into subclinical atherosclerotic disease induced or accelerated by chemotherapy and radiation. This review aims to highlight the use of cardiac CT in providing pre-treatment cardiovascular risk stratification for cardiotoxicity and evaluation of cardiac events during treatment and long-term surveillance, and reviews the evidence to date of utilizing this evolving technology in a unique population (Fig. 1). The utility of cardiac CT in evaluating primary and secondary malignancies involving the heart is beyond the scope of this document.
Fig. 1.
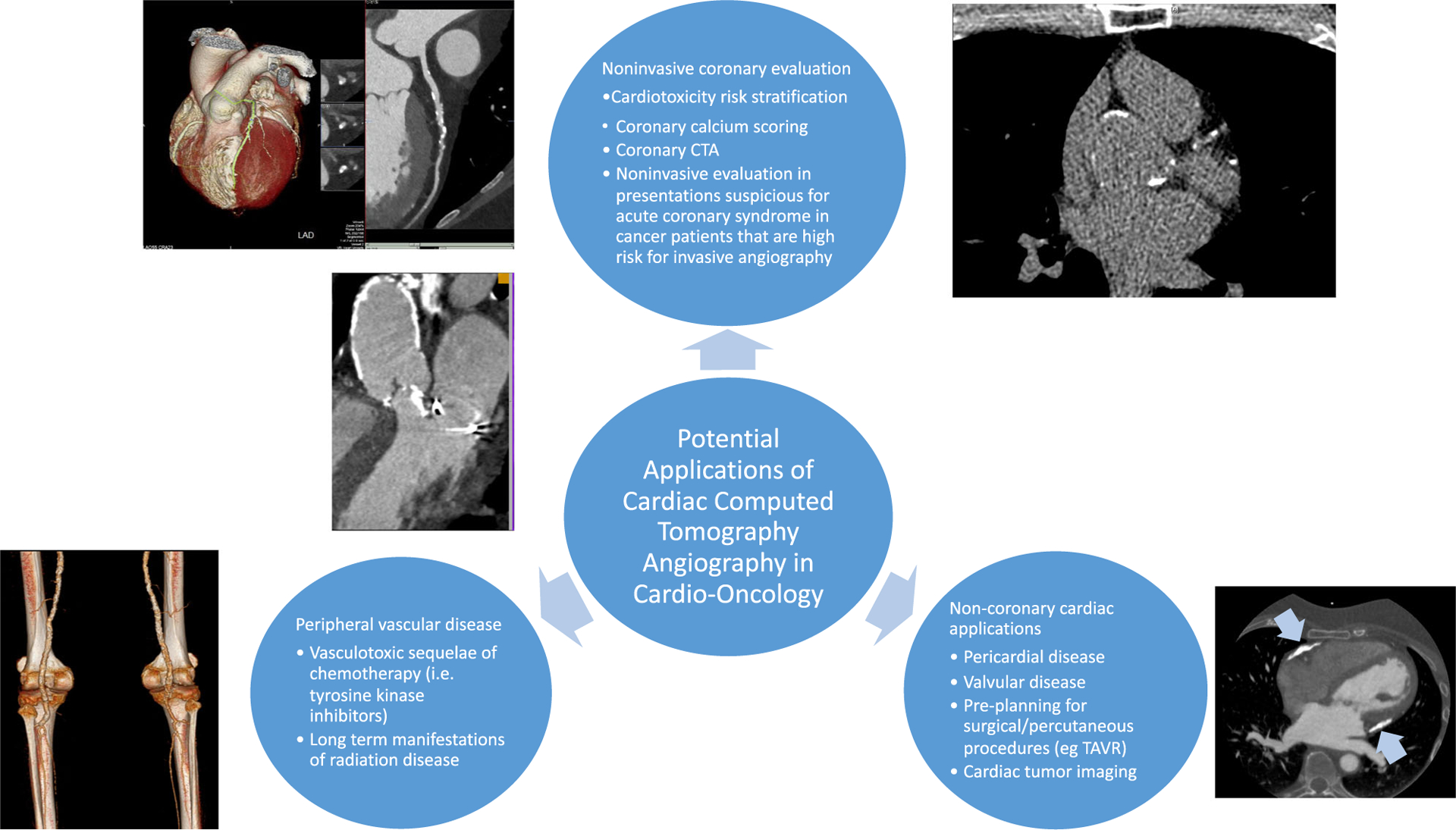
Potential applications of cardiac computed tomography in visualizing the entire spectrum of cardiovascular disease induced by cancer treatments.
Pre-treatment risk stratification
Mediastinal radiation and certain chemotherapy agents have known implications for cardiovascular disease. Along with anthracyclines, other chemotherapies including trastuzumab, fluoropyrimidines, cisplatin, taxanes, and tyrosine kinase inhibitors have all been linked with cardiovascular events [13]. With regards to radiation therapy, the effects on essentially all structures of the heart have been well documented in Hodgkin lymphoma and breast cancer survivors. The estimated cumulative incidence of radiation-induced heart disease is 10–30% 5 to 10 years after treatment [14]. The dose related to radiation treatments is measured in grey (Gy) units, which is the absorption of 1 J of ionizing radiation energy by 1 kg of tissue. Even minimal doses have been associated with long-term cardiovascular consequences, which is important in view of the improving radiation techniques over time with overall lower exposures [13, 15].
Although there have been improvements in radiotherapy techniques with the advent of 3-D conformal imaging, the development of intensity-modulated radiotherapy and proton therapy, the long-term sequela of these newer treatments are relatively unknown. In agreement with the historical perspective on this topic, the 2017 American Society of Clinical Oncology clinical practice guidelines has deemed radiotherapy in the area of the heart at doses ≥ 30 Gy in isolation, or at lower levels when combined with anthracycline and/or trastuzumab, to pose an increased risk for developing cardiac dysfunction [16]. While there has been much research looking at the incidence of cardiomyopathy, data assessing objective markers of atherosclerotic disease and its contribution to short- and long-term cardiotoxicity has been ill-defined.
Preexisting CAD and associated cardiovascular risk factors may increase the risk of cardiovascular events in those exposed to chemotherapy or radiation. This information dates back several decades with reports of a four-fold increase in cardiac events in patients with a cardiac history who received 5-fluorouracil, as well as significantly worsened left ventricular dysfunction in cardiac patients who receive anthracyclines [17, 18]. In lymphoma patients previously treated with radiation, cardiac risk factors pose an increased threat compared to a non-cardiac population [19]. Small molecule inhibitors—targeted therapies that have rapidly advanced onco-logic care—possess a side effect profile that may predispose patients with underlying atherosclerosis to worsening blood pressure control, peripheral artery disease (PAD), and even heart failure [20].
There remains a paucity of literature for pre-treatment risk stratification in those patients with underlying atherosclerotic burden. Traditionally, the focus has been on assessment of left ventricular function in patients receiving anthracyclines or trastuzumab. The 2016 Canadian Cardiovascular Society published pre-treatment guidelines for management of cardiovascular complications from chemotherapy included recommendations for strict blood pressure control and 12-lead ECGs prior to receiving QTc-prolonging agents [21]. Current recommendations, including the American Society of Echocardiography (ASE) and the European Association of Cardiovascular Imaging (EACVI), suggest the use of the same imaging modality (and preferably vendor) to monitor left ventricular function before, during, and after completion of cancer therapy, as well as evaluating myocardial strain for subclinical ventricular dysfunction [22]. However, to date, no formal guidelines exist for pre-treatment coronary evaluation and/or risk assessment.
Given the theoretical cardiotoxicity risk in patients with CAD, there is a need for pre-treatment screening tools. One such method is the coronary artery calcium (CAC) score (non-contrast cardiac CT scan). CAC scoring is an affordable and reproducible test (typical effective radiation dose of 1–1.5 mSv) that identifies calcium deposition in coronary vasculature [23]. Prior prospective, randomized trials have demonstrated asymptomatic individuals who underwent CAC had greater cardiac risk factor control without increased downstream medical testing compared to those who were assessed with clinical risk calculation alone [24]. Along with 10-year atherosclerotic cardiovascular disease risk assessment, CAC scoring in intermediate-risk patients may delineate those who benefit from primary prevention measures including lipid-lowering agents, aggressive diabetes control, and tighter blood pressure management per the updated AHA/ACC guidelines prior to receiving chemotherapy or radiation [25]. This has been corroborated in breast cancer patients in whom CAC scoring appears to serve as a marker for more aggressive risk reduction prior to treatment. A consecutive study was performed with 939 female breast cancer patients treated with radiotherapy, with CAC scores ranging from 0 to 2,859 (mean 27.3). The 9-year cumulative incidence of acute coronary events was 3.2%, with a significant association with the pre-treatment CAC score even after correcting for higher mediastinal radiation exposure. A high CAC score (≥ 400) had a hazard ratio of 4.95 (95% CI 1.69–14.53, p = 0.004) for acute coronary events compared to patients with a CAC score of 0 [26•]. Given its common role in cancer surveillance, non-ECG-gated chest CTs routinely obtained by oncologists can reliably detect CAC [27•]. Therefore, non-cardiac CT scans that are often acquired for the evaluation of cancer patients can be used to risk-stratify those patients. Recent guidelines of the Society of Cardiovascular CT support the role of CAC detection on non-cardiac scan, semiquantitative assessment of CAC burden, and use of these results for the guidance of preventative therapies [28]. Further, CAC scoring during lung cancer chest CT screening can serve as a marker to identify an at-risk population [29, 30]. While CAC scoring may have a role in evaluating patients treated with radiation therapy, limited data exist for its use as a screening tool prior to chemotherapy or radiation and prospective studies are needed to evaluate the impact of subclinical atherosclerosis on short- and long-term cardiotoxicity. In addition, recent retrospective data in high-risk populations, such as Hodgkin lymphoma, have shown a cumulative incidence of cardiovascular events in survivors at greater than 50%. Such a high event rate warrants more aggressive screening where traditional cardiovascular risk factor assessment may not apply [31]. Certain societies have advocated for the use of CAC/coronary CTA screening in cancer survivors, or in patients with suspected acute coronary syndrome who are at high risk of invasive coronary angiography-related complications, including the ASE/EACVI [32]. Further prospective research is warranted to assess if CAC scoring predicts cardiovascular events in the oncology patient population during treatment with cardiotoxic agents, and an increased cardiovascular risk in survivors compared to the general population and whether preventative therapies (e.g., statins) may improve cardiovascular and/or cancer outcomes.
Coronary CTA is a highly sensitive tool that provides superior imaging of coronary anatomy with increasingly shorter acquisition times and lower radiation exposure, particularly with multi-detector CT. Depending on the protocol used, radiation from coronary CTA is generally less than that of radionuclide imaging or invasive coronary angiography, with typically estimated radiation doses between 1 and 4 mSv using modern scanners and dose-reducing protocols. According to the 2014 ACC/AHA stable ischemic heart disease guidelines, it is a reasonable testing modality in intermediate-risk patients with an uninterpretable ECG who are unable to exercise, patients with prior equivocal stress test findings, or patients with persistent symptoms despite normal stress test results [33]. Its strength lies predominantly in its superior negative predictive value in excluding CAD. Moreover, in patients with equivocal stress imaging studies, CCTA may adjudicate use of invasive coronary angiography [34]. In an at-risk population who will receive chemotherapy or radiation, coronary CTA may be a reasonable alternative as a pre-treatment risk assessment tool. While the positive detection of CAD by coronary CTA should rarely preclude patients from receiving chemotherapy or radiation, it should drive cardio-oncologists to more aggressive secondary risk reduction strategies. To what extent pre-treatment coronary CTA would benefit, this patient population necessitates further investigation.
Monitoring for cardiovascular toxicity during treatment
A spectrum of cardiovascular toxicities with chemotherapy exists, and chest CT imaging may provide useful information regarding both heart and lung pathology, including pneumonitis, pulmonary embolism, pleural effusion/congestion, pericardial disease (effusion and metastases), acute coronary syndrome, valvular heart disease, and PAD. Along with the knowledge of a given chemotherapeutic agent’s cardiovascular toxicities, coronary CTA may benefit providers in early detection and diagnosis of these potential complications.
Several agents associated with acute coronary syndrome have been described above [4, 13, 18]. Following standard of care stable chest pain management per ACC/AHA guidelines, direct visualization of the coronary arteries by coronary CTA may be an appropriate alternative in low to moderate risk patients, especially given its high negative predictive value [35]. This is especially important as the recent 5-year outcomes data from the randomized Scottish computed tomography of the heart (SCOT-HEART) trial in non-cancer patients demonstrated that coronary CTA can decrease cardiac death and myocardial infarction by approximately 40% as compared to functional stress testing [36•]. In the setting of hematologic derangements (anemia, thrombocytopenia, coagulation abnormalities), invasive coronary angiography may be restricted secondary to bleeding risk, with coronary CTA being a reasonable option in this subset of patients presenting with cardiotoxic manifestations to exclude acute coronary syndrome. Angiographically intermediate appearing coronary lesions may benefit from developing technologies of functional coronary physiology, such as non-invasive CT-derived fractional flow reserve (FFRCT), to provide a non-invasive, informed risk-benefit guidance for intervention who may be at elevated risk for complication from repeated invasive procedures [37]. Along with direct visualization of the coronary vasculature to exclude CAD in patients presenting with conditions mimicking acute coronary syndrome, e.g., stress-induced cardiomyopathy, coronary CTA can also identify structural abnormalities such as valvular disease and intracardiac thrombosis.
Acute myocarditis, a potential sequela of immunotherapies—including, but not limited to high-dose interleukin-2 therapy and programmed death-ligand-1 inhibitors (immune checkpoint inhibitors)—can be diagnosed non-invasively with cardiovascular magnetic resonance late gadolinium enhancement and T2-weighted imaging. However, in those patients with contraindication to magnetic resonance imaging or gadolinium, coronary CTA may be a viable alternative. Delayed CT acquisition has demonstrated good correlation with cardiac magnetic resonance in areas of delayed enhancement, ranging from subendocardial ischemia and fibrosis to acute myocarditis. However, given that such protocols require iodinated contrast exposure and increased radiation exposure, the overall efficacy and benefit of this application of coronary CTA require further study [38].
Long-term CAD surveillance
Although premature atherosclerosis is a recognized sequela of some chemotherapies and radiation exposure, post-treatment CAD surveillance data has been inconsistent. The most commonly studied groups—breast cancer and Hodgkin lymphoma survivors—suffer from cardiovascular events with a relative risk two-to-five times greater than that of the general population [3, 39, 40••, 41]. The cause of which may be explained by a disproportionate effect of radiation on the ostia and proximal segments of the coronary arteries [42]. Traditional cardiac risk factors appear to synergistically contribute to CAD in this population [43••]. While radiotherapy regimens have shifted toward less radiation and tissue-specific targeting, even minimal exposure has been associated with increased major coronary events, which increases at a linear proportion to radiation exposure [39]. Further, these patients have increased rates of valvular disease and revascularization procedures, both of which portend a poorer prognosis than the general population [44].
Post-treatment cancer survivors with CAD can be asymptomatic, particularly in radiation survivors where autonomic dysfunction has been associated with mediastinal radiation [45]. Coronary CTA is an ideal surveillance tool for this population given its superior sensitivity to stress testing, especially for those with false-negative stress testing (e.g., balanced ischemia in nuclear myocardial perfusion imaging with ostial stenoses) that may benefit from aggressive risk reduction strategies. This was made apparent by Rademaker et al. who identified asymptomatic CAD in eight of nine previously treated Hodgkin lymphoma patients; subsequent to diagnosis by coronary CTA, one patient underwent percutaneous intervention and another coronary artery bypass surgery [46]. In a similar population, CAC volume scores greater than 200 correlated with known CAD, identifying a post-treatment role for screening with CAC scoring [31]. Coronary CTA has also been used in post-treatment surveillance; in one study, it identified significant CAD in 19 of 119 asymptomatic Hodgkin lymphoma patients; some of whom required revascularization following results of their scan [47].
The 2018 National Comprehensive Cancer Network guidelines for Hodgkin lymphoma survivors has stated that coronary CTA abnormalities are detectable in nearly 15% of the patients within the first 5 years after treatment, and their incidence significantly increases 10 years after treatment. Furthermore, they advise stress testing/echocardiography at 10-year intervals after treatment without mention of direct CAD monitoring [48]. An ASE/EACVI consensus statement recommends stress testing every 5 years after radiation therapy to the chest in high-risk cancer patients [32]. Regarding age cut-offs, Heidenreich et al. and Van Leeuwen-Segarceanu et al. recommend commencing CAD screening with CAC scoring in Hodgkin lymphoma survivors 5 years out from radiation therapy if older than 45 years of age and starting 10 years out if younger [41, 45]. The rationale for screening frequency screening is driven by the risk of accelerated atherosclerosis in mediastinal radiation patients with CAD reported as early as one year out from therapy [45]. At present, these are conceptually attractive strategies that require translation in modifying disease progression.
Long term radiation-induced valvulopathy
Valvular disease is an often-overlooked long-term complication of mediastinal radiation. Mitral and aortic valves are most commonly affected, resulting in either insufficiency or stenosis, with symptomatology ranging from mild to severe [3]. The reported prevalence of valve dysfunction ranges from 5–40% and typically presents 15 to 20 years after initial radiation exposure [49]. These patients are often younger at time of valve replacement, and undergo valve surgery more often than the general population [50]. Anthracyclines, a cornerstone for Hodgkin lymphoma treatment, may also pose an additive risk to valve dysfunction [51].
Echocardiography is the standard of care for non-invasive valve screening in post-treatment Hodgkin lymphoma patients. Some groups including the ASE/EACVI recommend screening starting at 10 years out from mediastinal radiation and repeating every 5 years, or at onset of cardiovascular complaints [13, 41]. Given the potential hemodynamic sequela of end-stage valvular disease, there is a need for a more sensitive screening tool, especially when echocardiographic images are suboptimal secondary to body habitus or hyperinflated lungs. Cardiac CTA may fill this void as a reliable alternative for valve assessment as it is not dependent on the operator or acoustic window shadowing [50]. Further, radiation-induced valvular disease rarely exists in isolation and may also be associated with premature CAD and/or radiation-induced constrictive pericarditis (CT is effective in detecting calcific deposits in the pericardium), allowing for surveillance of several disease processes with one imaging modality. If there is a need for surgical or transcatheter valve replacement, preoperative coronary CTA may negate the need for invasive coronary angiography given its high negative predictive value for excluding obstructive CAD, as well as providing adjunctive valve dimension, valve positioning information, and vascular anatomy prior to the procedure. Further, cardiac CT is essential in pre-procedural planning of transcatheter aortic valve replacement (TAVR) [52]. In those patients with known malignancy, TAVR for severe aortic stenosis is associated with improved survival, regardless of cancer type [53]. In a worldwide registry evaluating 222 patients with active malignancy undergoing TAVR, patients had similar short term but worse long-term prognosis, with mortality mainly being driven by cancer. It is notable that at 1 year, up to one third of patients were in remission or cured of their cancer, with TAVR likely being an intervention allowing them to proceed with their cancer treatments [54]. Thus, pre-procedural imaging with CCTA may provide anatomic information of cardiac and vascular disease not only for the structural intervention, but also provide information that may drive long-term cardiovascular treatments to attenuate long-term risk of cardiac events, especially with patients who have a good long-term prognosis with their cancer. Other affected organs from radiotherapy may also be appreciated involving the aorta, lungs, and other cardiac structures, including the pericardium.
Peripheral artery disease from vasculotoxic therapies
Several small molecule inhibitors and radiotherapy in the abdomen and lower extremities have been linked with accelerated progression of PAD, especially in those patients with underlying preexisting cardiovascular risk factors [55–58]. Nilotinib and ponatinib—two tyrosine kinase inhibitors used for the treatment of Philadelphia chromosome-positive chronic myelocytic leukemia—have been associated with rapidly progressive peripheral atherosclerosis, sometimes resulting in early termination of crucial therapy [58, 59]. Several retrospective analyses have reported occlusive PAD events following ponatinib and nilotinib administration in as many as 40% and 25%, respectively. The pathophysiology of this mechanism is likely related to endothelial cell injury and aggravated in those with preexisting vascular disease [59]. Risk stratification should be considered for preexisting PAD prior to initiation of such high-risk treatments. If there is a concern for vascular claudication symptoms, a thorough history and physical exam should guide further evaluation by performing ankle-brachial indices and possibly peripheral CTA with distal runoff prior to initiating therapy. Given the concomitant risk of CAD, detection and subsequent treatment of PAD may also provide opportunity to prevent future coronary events.
Future directions
With the current advances in cancer-targeted therapy and lower, more specific radiation dosing, the optimal screening for asymptomatic cardiac disease is unknown. Extrapolating screening recommendations by the aforementioned groups to others treated with cardiotoxic chemotherapy and radiation regimens requires prospective analysis, with cardiac CT/coronary CTA being a viable screening option for subclinical/clinical atherosclerotic disease. In addition, there should be a low threshold for evaluation of potential cardiac symptoms in high-risk cancer survivors and that routine counseling and surveillance are indicated after Hodgkin lymphoma therapy to ensure timely interventions. For patients with multiple cardiovascular risk factors, early screening for CAD before initiation of chemotherapy or radiation may optimize cardiovascular therapy with the goal of preventing premature discontinuation of vital cancer treatment. The assessment of non-cardiac CT scan should include detection and CAC, and preventative therapies (e.g., statins) should be considered in patients with evidence of coronary atherosclerosis. Coronary CTA can also provide a non-invasive imaging modality in excluding or evaluating for atherosclerotic disease in patients undergoing treatments who develop cardiotoxic reactions that may mimic acute coronary syndrome but are too high risk to undergo invasive angiography. Finally, detection of CAD in cancer survivors also poses management dilemmas in asymptotic individuals. Along with initiating ACC/AHA guideline–directed therapy, novel lipid-lowering agents—e.g., PCSK9 inhibitors—may be a focus of interest in this susceptible subset of patients. With the increasing worldwide prevalence of cancer survivors, improving cardiovascular health through preventative screening initiatives, potentially with the utility of coronary CTA, is the next chapter for this aging population.
Conflict of Interest
Michael E. Layoun declares that he has no conflict of interest.
Eric H. Yang declares that he has no conflict of interest.
Joerg Herrmann declares that he has no conflict of interest.
Cezar A. Iliescu declares that he has no conflict of interest.
Juan C. Lopez-Mattei declares that he has no conflict of interest.
Kostas Marmagkiolis declares that he has no conflict of interest.
Matthew J. Budoff has received research funding from General Electric.
Maros Ferencik has received research funding from the American Heart Association and the National Institutes of Health.
Footnotes
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “silver tsunami”: prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers. 2016;25(7):1029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Groarke JD, Nguyen PL, Nohria A, Ferrari R, Cheng S, Moslehi JJ. Cardiovascular complications of radiation therapy for thoracic malignancies: the role for non-invasive imaging for detection of cardiovascular disease. Eur Heart J. 2014;35(10):612–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moslehi JJ. Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med. 2016;375(15):1457–67. [DOI] [PubMed] [Google Scholar]
- 5.Potts JE, Kwok CS, Rashid M, Mamas MA, Iliescu CA, Lopez Mattei JC, et al. Percutaneous coronary intervention in cancer patients: a report of the prevalence and outcomes in the United States. 2018 [DOI] [PubMed]
- 6.Armstrong GT, Oeffinger KC, Chen Y, Kawashima T, Yasui Y, Leisenring W, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31(29):3673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011;13(3):R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dehal AN, Newton CC, Jacobs EJ, Patel AV, Gapstur SMCP. Impact of diabetes mellitus and insulin use on survival after colorectal cancer diagnosis: the Cancer Prevention Study-II Nutrition Cohort. J Clin Oncol. 2012;1(30):53–9. [DOI] [PubMed] [Google Scholar]
- 9.Abdel-Qadir H, Austin PCLD. A population-based study of cardiovascular mortality following early-stage breast cancer. JAMA Cardiol. 2017;2(1):88–93. [DOI] [PubMed] [Google Scholar]
- 10.Whitlock MC, Yeboah J, Burke GL, Chen H, Klepin HD, Hundley WG. Cancer and its association with the development of coronary artery calcification: an assessment from the multi-ethnic study of atherosclerosis. J Am Heart Assoc. 2015;4(11):e002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hershman DL, Till C, Shen S, Wright JD, Ramsey SD, Barlow WE, et al. Association of cardiovascular risk factors with cardiac events and survival outcomes among patients with breast cancer enrolled in SWOG clinical trials. J Clin Oncol. 2018;36(26):2710–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh J, Valero-Elizondo J, Salami JA, Warraich HJ, Ogunmoroti O, Spatz ES, et al. Favorable modifiable cardiovascular risk profile is associated with lower healthcare costs among cancer patients: the 2012–2013 medical expenditure panel survey. J Am Heart Assoc. 2018;7(9):e007874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herrmann J, Yang EH, Iliescu CA, Cilingiroglu M, Charitakis K, Hakeem A, et al. Vascular toxicities of cancer therapies: the old and the new—an evolving avenue. Circulation. 2016;133(13):1272–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carver JR, Shapiro CL, Ng A, Jacobs L, Schwartz C, Virgo KS, et al. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol. 2007;25(25):3991–4008. [DOI] [PubMed] [Google Scholar]
- 15.Russell RR, Alexander J, Jain D, Poornima IG, Srivastava AV, Storozynsky E, et al. The role and clinical effectiveness of multimodality imaging in the management of cardiac complications of cancer and cancer therapy. J Nucl Cardiol. 2016;23:856–84. [DOI] [PubMed] [Google Scholar]
- 16.Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2017;35:893–911. [DOI] [PubMed] [Google Scholar]
- 17.Labianca R, Beretta G, Clerici M, Fraschini PLG. Cardiac toxicity of 5-FU: a study of 1,083 patients. Tumori. 1982;68:505–10. [DOI] [PubMed] [Google Scholar]
- 18.Layoun ME, Wickramasinghe CD, Peralta MV, Yang EH. Fluoropyrimidine-induced cardiotoxicity: manifestations, mechanisms, and management. Curr Oncol Rep. 2016;18:35. [DOI] [PubMed] [Google Scholar]
- 19.Glanzmann C, Kaufmann P, Jenni R, Hess OM, Huguenin P. Cardiac risk after mediastinal irradiation for Hodgkin’s disease. Radiother Oncol. 1998;46(1):51–62. [DOI] [PubMed] [Google Scholar]
- 20.de Jesus-Gonzalez N, Robinson E, Moslehi J, Humphreys BD. Management of antiangiogenic therapy-induced hypertension. Hypertension. 2012;60(3):607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Virani SA, Brezden-Masley C, Clarke B, Davis MK, Jassal DS, Johnson C, et al. Canadian cardiovascular society guidelines for evaluation and management of cardiovascular complications of cancer therapy. Can J Cardiol. 2016;32:831–41. [DOI] [PubMed] [Google Scholar]
- 22.Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27(9):911–39. [DOI] [PubMed] [Google Scholar]
- 23.Kim KP, Einstein AJ, Berrington de González A. Coronary artery calcification screening: estimated radiation dose and cancer risk. Arch Intern Med. 2009;169(13):1188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rozanski A, Gransar H, Shaw LJ, Kim J, Miranda-Peats L, Wong ND, et al. Impact of coronary artery calcium scanning on coronary risk factors and downstream testing: the EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) prospective randomized trial. J Am Coll Cardiol. 2011;57(15):1622–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol. J Am Coll Cardiol 2018;25709. [Google Scholar]
- 26.•.Roos CTG, van den Bogaard VAB, Greuter MJW, Vliegenthart R, Schuit E, Langendijk JA, et al. Is the coronary artery calcium score associated with acute coronary events in breast cancer patients treated with radiotherapy? Radiother Oncol. 2018;126(1):170–6. [DOI] [PubMed] [Google Scholar]; Elevated coronary artery calcium scores are associated with higher cardiovascular event rates in breast cancer patients.
- 27.•.Hughes-Austin JM, Dominguez A 3rd, Allison MA, Wassel CL, Rifkin DE, Morgan CG, et al. Relationship of Coronary Calcium on Standard Chest CT Scans With Mortality. JACC Cardiovasc Imaging. 2016;9(2):152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]; Oncologists can use non-ECG gated chest CT coronary artery calcium scores to assist in cardiovascular risk stratification.
- 28.Hecht HS, Blaha MJ, Kazerooni EA, Cury RC, Budoff M, Leipsic J, et al. CAC-DRS: coronary artery calcium data and reporting system. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT). J Cardiovasc Comput Tomogr. 2018;12(3):185–91. [DOI] [PubMed] [Google Scholar]
- 29.Garg PK, Jorgensen NW, McClelland RL, Leigh JA, Greenland P, Blaha MJ, et al. Use of coronary artery calcium testing to improve coronary heart disease risk assessment in a lung cancer screening population: the Multi-Ethnic Study of Atherosclerosis (MESA). J Cardiovasc Comput Tomogr. 2018;12(6):493–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu MT, Onuma OK, Massaro JM, D’Agostino RB, O’Donnell CJ, Hoffmann U. Lung cancer screening eligibility in the community: cardiovascular risk factors, coronary artery calcification, and cardiovascular events. Circulation. 2016;134(12):897–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andersen R, Wethal T, Günther A, Fosså A, Edvardsen T, Fosså SD, et al. Relation of coronary artery calcium score to premature coronary artery disease in survivors 915 years of Hodgkin’s lymphoma. Am J Cardiol. 2010;105(2):149–52. [DOI] [PubMed] [Google Scholar]
- 32.Lancellotti P, Edvardsen T, Gaemperli O, Galderisi M, Griffin B, Plana JC, et al. Expert consensus for multimodality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging. 2013;14(8):721–40. [DOI] [PubMed] [Google Scholar]
- 33.Amsterdam EA, et al. AHA/ACC guideline for the management of patients with non–ST-elevation acute coronary syndromes. Circulation. 2014;130(25):e344–426. [DOI] [PubMed] [Google Scholar]
- 34.Chinnaiyan KM, Raff GL, Goraya T, Ananthasubramaniam K, Gallagher MJ, Abidov A, et al. Coronary computed tomography angiography after stress testing: results from a multicenter, statewide registry, ACIC (Advanced Cardiovascular Imaging Consortium). J Am Coll Cardiol. 2012;59(7):688–95. [DOI] [PubMed] [Google Scholar]
- 35.Litt HI, Gatsonis C, Snyder B, Singh H, Miller CD, Entrikin DW, et al. CT angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med. 2012;366(15):1393–403. [DOI] [PubMed] [Google Scholar]
- 36.•.Investigators S-H, Newby DE, Adamson PD, Berry C, Boon NA, Dweck MR, et al. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med. 2018;379:924–33. [DOI] [PubMed] [Google Scholar]; The SCOT-HEART investigators provided insight into the mortality benefit of CCTA in a stable chest pain population.
- 37.Douglas PS, Pontone G, Hlatky MA, Patel MR, Norgaard BL, Byrne RA, et al. Clinical outcomes of fractional flow reserve by computed tomographic angiography-guided diagnostic strategies vs. usual care in patients with suspected coronary artery disease: the prospective longitudinal trial of FFRct: outcome and resource impacts stud. Eur Heart J. 2015;36(47):3359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Redheuil AB, Azarine A, Garrigoux P, Mousseaux E. Correspondence between delayed enhancement patterns in multislice computed tomography and magnetic resonance imaging in a case of acute myocarditis. Circulation. 2006;114(19):e571–2. [DOI] [PubMed] [Google Scholar]
- 39.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987–98. [DOI] [PubMed] [Google Scholar]
- 40.••.van Nimwegen FA, Schaapveld M, Janus CPM, Krol ADG, Petersen EJ, Raemaekers JMM, et al. Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Intern Med. 2015;175(6):1007–17. [DOI] [PubMed] [Google Scholar]; Van Nimwegen et al. provided extensive longitudinal efforts in defining long-term cardiovascular risk in a Hodgkin lymphoma population.
- 41.van Leeuwen-Segarceanu EM, Bos WJW, Dorresteijn LDA, Rensing BJWM, van d HJAS, Vogels OJM, et al. Screening Hodgkin lymphoma survivors for radiotherapy induced cardiovascular disease. Cancer Treat Rev. 2011;37(5):391–403. [DOI] [PubMed] [Google Scholar]
- 42.Chinnasami BR, Schwartz RC, Pink SB, Skotnicki RA. Isolated left main coronary stenosis and mediastinal irradiation. Clin Cardiol. 1992;15:459–61. [DOI] [PubMed] [Google Scholar]
- 43.••.Chao C, Xu L, Bhatia S, Cooper R, Brar S, Wong FL, et al. Cardiovascular disease risk profiles in survivors of adolescent and young adult (AYA) cancer: the Kaiser Permanente AYA Cancer Survivors study. J Clin Oncol. 2016;34(14):1626–3. [DOI] [PubMed] [Google Scholar]; Chao et al. confirmed the increased risk of cardiovascular disease in cancer population compared to the general public, especially in those with prior leukemia or breast cancer.
- 44.Hull MC, Morris CG, Pepine CJ, et al. Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of Hodgkin lymphoma treated with radiation therapy. JAMA. 2003;290:2831–7. [DOI] [PubMed] [Google Scholar]
- 45.Heidenreich PA, Schnittger I, Strauss HW, Vagelos RH, Lee BK, Mariscal CS, et al. Screening for coronary artery disease after mediastinal irradiation for Hodgkin’s disease. J Clin Oncol. 2007;25(1):43–9. [DOI] [PubMed] [Google Scholar]
- 46.Rademaker J, Schroder H, Ariaratnam NS, Strauss HW, Yahalom J, Steingart R, et al. Coronary artery disease after radiation therapy for Hodgkin’s lymphoma: coronary CT angiography findings and calcium scores in nine asymptomatic patients. Am J Roentgenol. 2008;191(1):32–7. [DOI] [PubMed] [Google Scholar]
- 47.Küpeli S, Hazirolan T, Varan A, Akata D, Alehan D, Hayran M, et al. Evaluation of coronary artery disease by computed tomography angiography in patients treated for childhood Hodgkin’s lymphoma. J Clin Oncol. 2010;28(6):1025–30. [DOI] [PubMed] [Google Scholar]
- 48.The National Comprehensive Cancer. NCCN guidelines version 3.2018. Hodgkin lymphoma. Version 3.2018, 08/31/18 © National Comprehensive Cancer Network, Inc. 2018, All rights reserved. [Google Scholar]
- 49.Dijos M, Reynaud A, Leroux L, Réant P, Cornolle C, Roudaut R, et al. Efficacy and follow-up of transcatheter aortic valve implantation in patients with radiation-induced aortic stenosis. Open Hear. 2015;2(1):e000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bouvier E, Logeart D, Sablayrolles JL, Feignoux J, Scheublé C, Touche T, et al. Diagnosis of aortic valvular stenosis by multislice cardiac computed tomography. Eur Heart J. 2006;27:3033–8. [DOI] [PubMed] [Google Scholar]
- 51.Buttan AK, Yang EH, Budoff MJ, Vorobiof G. Evaluation of valvular disease by cardiac computed tomography assessment. J Cardiovasc Comput Tomogr. 2012;6(6):381–92. [DOI] [PubMed] [Google Scholar]
- 52.Blanke P, Weir-McCall JR, Achenbach S, Delgado V, Hausleiter J, Jilaihawi H, et al. Computed tomography imaging in the context of transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR): an expert consensus document of the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr. 2019;13:1–20. [DOI] [PubMed] [Google Scholar]
- 53.Schechter M, Balanescu DV, Donisan T, Dayah TJ, Kar B, Gregoric I, et al. An update on the management and outcomes of cancer patients with severe aortic stenosis. Catheter Cardiovasc Interv 2018;1–8. [DOI] [PubMed] [Google Scholar]
- 54.Landes U, Iakobishvili Z, Vronsky D, Zusman O, Barsheshet A, Jaffe R, et al. Transcatheter aortic valve replacement in oncology patients with severe aortic stenosis. JACC Cardiovasc Interv. 2019;12(1):78–86. [DOI] [PubMed] [Google Scholar]
- 55.Pollak AW, Norton P, Kramer CM. Multimodality imaging of lower extremity peripheral arterial disease: current role and future directions. Circ Cardiovasc Imaging. 2012;5(6):797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.El Tahlawi M, Jop B, Bonello B, Dragulescu A, Rouault F, Habib G, et al. Should we close hypoxaemic patent foramen ovale and interatrial shunts on a systematic basis. Arch Cardiovasc Dis. 2009;102(11):755–9. [DOI] [PubMed] [Google Scholar]
- 57.Kim TD, Rea D, Schwarz M, Grille P, Nicolini FE, Rosti G, et al. Peripheral artery occlusive disease in chronic phase chronic myeloid leukemia patients treated with nilotinib or imatinib. Leukemia. 2013;27:1316–21. [DOI] [PubMed] [Google Scholar]
- 58.Aichberger KJ, Herndlhofer S, Schernthaner G-H, Schillinger M, Mitterbauer-Hohendanner G, Sillaber C, et al. Progressive peripheral arterial occlusive disease and other vascular events during nilotinib therapy in CML. Am J Hematol. 2011;86(7):533–9. [DOI] [PubMed] [Google Scholar]
- 59.Cortes JE, Kim D, Pinilla-Ibarz J, le Coutre P, Paquette R, Chuah C, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369(19):1783–96. [DOI] [PMC free article] [PubMed] [Google Scholar]


