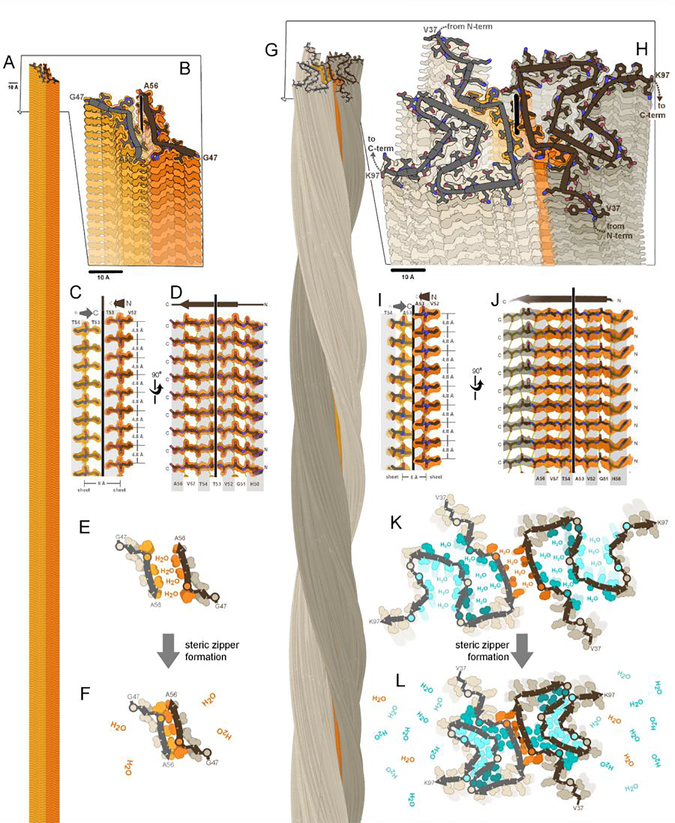
Figure 1. A fundamental component of amyloid fibrils is the steric zipper motif.
Amyloid fibrils of α-synuclein are associated with Parkinson’s disease and other synucleinopathies. The crystal structure of residues 47–56 offered atomic resolution details of its assembly at 1.4 Å resolution (PDB ID 4ZNN)(Rodriguez et al., 2015). (A) Thousands of copies of this segment stack to form a pair of β-sheets (light and dark orange). (B) A view oblique to the fibril axis reveals the tight fit between side chains of the mated sheets. (C) A view perpendicular to the fibril axis (vertical line) reveals that this zipper-like mating of side chains extends along the entire length of the fibril. (D) The remaining orthogonal view shows how the β-strands stack into β-sheets via main chain hydrogen bonds (dotted lines), parallel and in-register. Amide C=O and N-H groups point up and down, nearly parallel to the fibril axis. (E,F) Steric zipper formation liberates protein-bound water molecules, contributing a hydrophobic effect to fibril stability. (G) As determined by cryoEM at 2.8 resolution (Ni et al., 2019), full-length α-synuclein molecules stack in hydrogen-bonded sheets like the segment, but with a slight twist of each molecule compared to the molecule 4.8 Å below (PDB ID 6osj). Two protofilaments (brown and gray) intertwine to form a fibril. The preNAC region, is colored orange. (H) A view oblique to the fibril axis reveals each molecule is confined to a nearly flat layer. Each chain adopts the same meandering path comprising a series of β-strands and turns which mate together side chains in steric zippers. The steric zipper motif bridging the two protofilaments (orange) is analogous to that of the segment in panel B. (I,J) Views perpendicular to the fibril axis reveal steric zipper interactions and hydrogen bonding patterns analogous to the segment in panels C and D. (K,L) Assembly of 7 steric zippers (orange and cyan shades) liberates water molecules, a process that contributes greatly to amyloid stability.