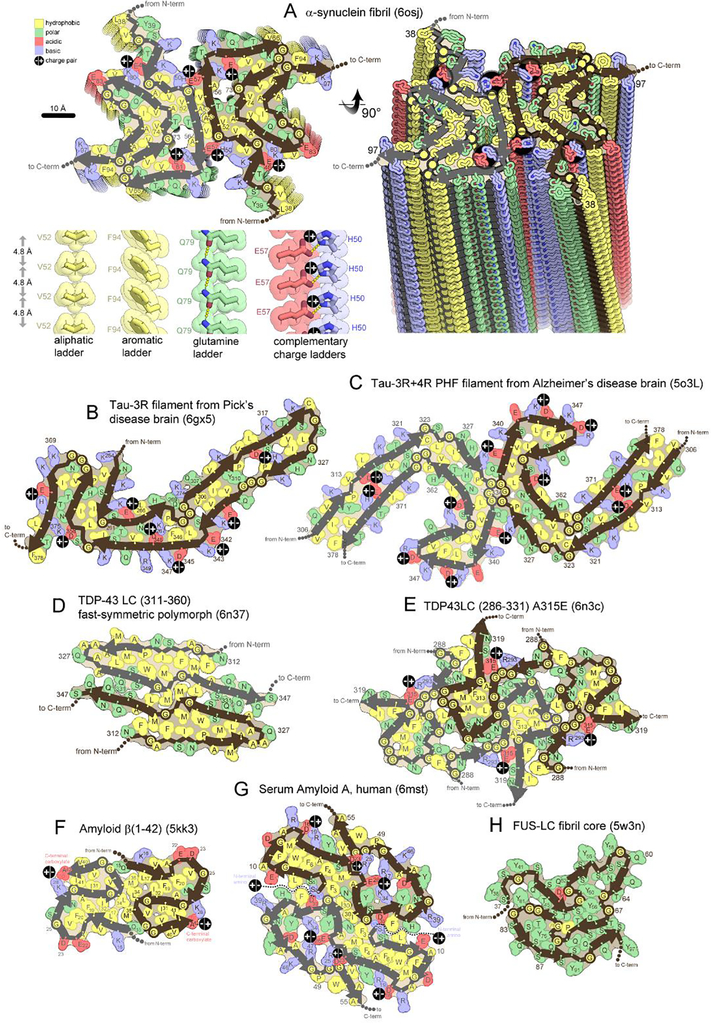
Figure 2. Folding patterns of amyloid proteins in compact, 2D layers.
(A) 2D layers of α-synuclein (upper left) stack into a fibril with a slight twist between layers. Thick lines trace the protein backbone. Brown and gray lines distinguish separate protofilaments. Sidechains are colored according to physical property. A view perpendicular to the fibril axis (right) reveals that identical residues stack in ladders along the fibril axis. Four types of amino acid ladders are depicted in detail (lower left), revealing stabilizing van der Waals contacts and/or hydrogen bonding and/or charge complementation. (B-H) Two dimensional layers of seven diverse amyloid fibrils reveal patterns of side chain association akin to those in globular proteins; hydrophobic residues (yellow) cluster together and tend to be buried. Polar residues also cluster together or reside on the surface. Charged residues associate in complementary pairs (black circle with +/− inscribed) or reside on the surface. β-arches (main chain U-turns) are ubiquitous. Steric zippers (extended β-strands running side-by-side) are evident in all but panel E and H.