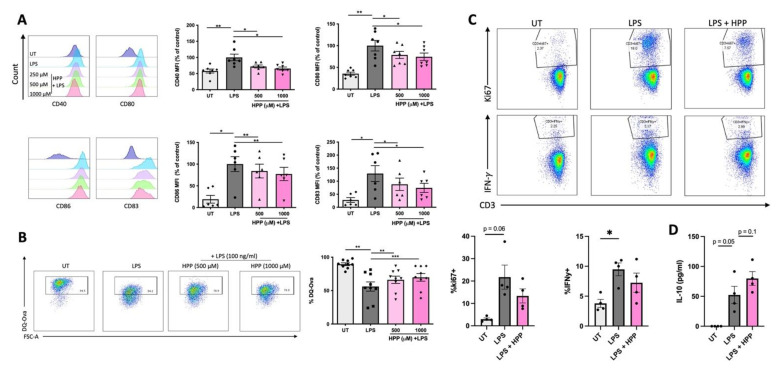
Figure 4.
HPP treatment reduces DC maturation and subsequent CD4+ T cell activation. Primary human DC were left untreated (UT) or incubated with HPP (500–1000 μM) for 6 h prior to stimulation with LPS (100 ng/mL) for 24 h. (A) Cells were stained for CD40, CD80, CD86, and CD83 and analysed by flow cytometry. Histograms showing the expression of maturation markers for HPP-treated, LPS-stimulated DC compared to unstimulated cells or LPS stimulation alone from one representative experiment. Pooled data showing the mean (±SEM) MFI for each marker expressed as a percentage of control (LPS stimulation alone) from six to seven healthy donors. (B) DC were incubated with FITC-conjugated DQ-Ovalbumin (DQ-Ova; 500 ng/mL) for 20 min and were immediately acquired by flow cytometry. Dot plots depicting DQ-Ova uptake from one representative experiment. Pooled data showing the mean (±SEM) DQ-Ova uptake as a percentage of total cells from nine healthy donors. (C) DC were pre-treated with HPP prior to stimulation with LPS, and subsequently cultured with CD4+ T cells for five days. Dot plots depicting ki67 expression (as a measure of proliferation) and IFNγ expression from one representative experiment. Pooled data showing the mean (±SEM) of ki67+ and IFNγ+ cells as a percentage of CD3+CD8− cells from four healthy donors. (D) Cell supernatants were assessed for IL-10 secretion by ELISA. Pooled data depict mean (±SEM) cytokine concentrations for four healthy donors (means of three technical replicates per donor). Repeated measures one-way ANOVA, with Dunnett’s multiple comparisons post hoc test, was used to determine statistical significance by comparing means of treatment groups against the mean of the control group (*** p < 0.001, ** p < 0.01, * p < 0.05).