Figure 2.
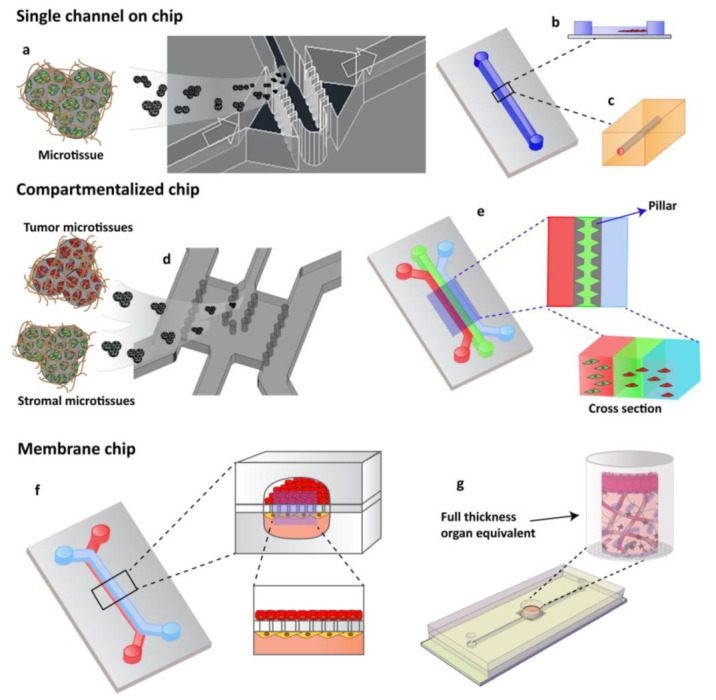
OOCs designs with different cell culture options. (a) Single channels chip designed by Garziano et al. to induce the assembly of dermal microtissues and on-line monitoring the newly synthesized collagen network, by means of SHG imaging, allowing to quantify in real time the effect of perfusion flow and biochemical stimulation on the newly collagen assembly degree. Reproduced from [96] with permission from The Royal Society of Chemistry. (b) Lumen channel designed to model human breast cancer cells collective invasion from primary tumors in response to interstitial fluid pressure, as reported by Piotrowski-Daspit et al. [87] or (c) designed to mimic a microvessel within a collagen gel scaffold as reported by Pauty et al. to study VEGF-A-induced angiogenesis permeability and angiogenic inhibitors effects [76]. (d,e) Compartmentalized chip: In these devices, pillars are used to separate microchannels in which 3D cell culturing is possible. The microchannels are independently addressable in order to fill each channel with a specific cell population and to allow heterotypic cultures. Depending upon the design of the chip, a 3D cell culture can occur by injecting the specific cell populated hydrogel in the correspondent channel or filling the microchamber with specific 3D microtissue. (d) The micropillar guarantees the physical contact of different cellular species embedded in their own ECM [93] or (e) in an ECM-like matrix. (f,g) Membrane chip. These devices allow a co-culture in a series of microchannels/chambers separated by a porous membrane. This multi-layered chip type was originally developed to mimic the endo- and epithelial cell layers found in the lung by Ingber [24]. Further, different devices have been designed in order to perform 3D organotypic culture by using both (f) membrane or (g) transwell insert.
