Abstract
As a significant chromosomal structural abnormality, chromosomal inversion is closely related to male infertility. For inversion carriers, the interchromosomal effect explains male infertility, but its specific mechanism remains unclear. Additionally, inversion carriers with different chromosomes have different clinical manifestations. Therefore, genetic counseling is difficult in clinical practice. Herein, four male carriers of pericentric inversion in chromosome 6 have been described. Two patients showed asthenospermia, one showed azoospermia, and the wife of the remaining patient had recurrent miscarriages. Through a literature search, the association between the breakpoint of pericentric inversion in chromosome 6 and male fertility problems are also discussed in this study. Overall, important genes related to asthenospermia in chromosome 6p21 were found, which may be related to the clinical phenotype. These results suggest that physicians should focus on the breakpoints of inversion in genetic counseling.
Keywords: male infertility, pericentric inversion, chromosome 6, genetic counseling
1. Introduction
Male infertility is a common clinical problem reported to reproductive medicine centers. Male factors contribute to approximately 50% of all infertility among couples [1]. Genetic variation is one of the important causes of male infertility. Recently, Tang et al. [2] reported that variants of homozygous helicase for meiosis 1 are responsible for spermatogenic failure. MLH3 single nucleotide polymorphisms in human populations can lead to male infertility [3,4,5]. The breakpoints of chromosome structural rearrangement could result in additional alterations, such as gene disruption or position effect, which ultimately lead to male infertility [6]. Hence, karyotype analysis is an important component of male infertility analysis [7]. Chromosomal aberrations include structural and numerical abnormalities. Structural abnormalities may affect spermatogenesis and are closely associated with male infertility [8]. Inversion is an important structural aberration and has been proven to be associated with male infertility [8,9,10,11].
The specific mechanisms underlying the effects of inversion chromosome on fertility is still unknown. This is because accurate genetic counseling remains challenging for the inversion carrier. For the carriers with chromosomal structural abnormality, preimplantation genetic diagnosis (PGD) has been recommended to give birth to offspring, as it has been proven to improve live birth rates and decrease miscarriage rates [12,13]. However, recent studies have shown that PGD cannot provide the same prominent benefits for inversion carriers in the Chinese Han population [14]. Moreover, a few cases of familial pericentric inversion have been reported [9,15,16]. Although these inversion carriers have a history of adverse pregnancy, they can still conceive naturally. Natural pregnancy can also be used as an option for inversion carriers [10].
This study reported four males with chromosome 6 inversion. Moreover, the association between pericentric inversion of chromosome 6 and male fertility problems has been discussed considering published cases as well.
2. Materials and methods
This study was approved by the Ethics Committee of the Second Hospital, Jilin University. Written informed consent has been obtained from all participants for the publication of these cases.
2.1. Patients
The patients included here had visited the andrology outpatient department of the Second Hospital, Jilin University, China. A questionnaire survey was conducted to collect patient data, such as age, marriage status, pregnancy history, genetic family history, anamnesis information, smoking and drinking history, and intervention of drugs. Physical examination was performed to record patients’ height, weight, growth and development information, and testicular size. We included four male carriers with chromosome 6 inversion.
2.2. Semen analysis
After abstinence for 3–7 days, patients’ semen was collected in a sterile container and examined by two professional technicians after liquefaction. Semen parameters were detected using the computer-aided semen analysis system (Beion S3, Shanghai Beion Medical Technology Co., Ltd, Shanghai, China). Azoospermia was diagnosed when no sperm was detected in the semen after centrifuging the sample three times and after excluding nonejaculation and retrograde ejaculation. Asthenozoospermia was diagnosed when the percentage of progressive sperm in semen was lower than the reference value of 32%.
2.3. Cytogenetic analysis
Peripheral blood (2 mL) was collected from all patients in sterile tubes containing heparin anticoagulant. Lymphocytes were cultured in RPMI-1640 culture medium (including phytohemagglutinin) (Yishengjun; Guangzhou Baidi Biotech, Guangzhou, China) for 72 h. Then, G-banding was performed using standard operating procedure. At least 20 metaphases were analyzed for each patient. The karyotypes were described according to the International System for Human Cytogenetic Nomenclature (ISCN 2016).
2.4. Collection of published cases
To explore the relationship between chromosome 6 inversion and male fertility problems, a PubMed search was performed using the keywords “chromosome/inversion/male infertility.” In addition, the database (literature we have read) was analyzed, and cases on chromosomal 6 inversion were collected. We included the cases of chromosomal 6 inversion in adult males; prenatal diagnosis cases were excluded. Moreover, related genes on chromosome 6 were searched using Online Mendelian Inheritance in Man (OMIM; https://www.ncbi.nlm.nih.gov/omim).
3. Results
3.1. Patient characteristics
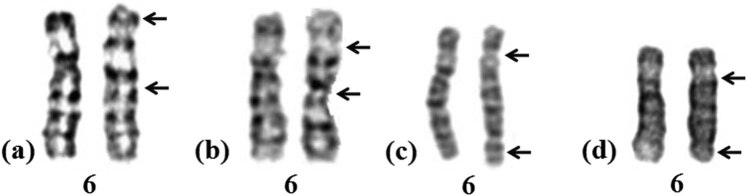
Case 1 involved a 29-year-old man who presented with normal intelligence and phenotype. He visited the hospital for consultation because his wife had two spontaneous miscarriages. Semen analysis showed that semen parameters were within the normal range. Cytogenetic analysis revealed that his karyotype was 46,XY,inv(6)(p23q13) (Figure 1a) and that of his wife was 46,XX. Routine gynecological examination showed that his wife had a normal reproductive function.
Figure 1.
G-banding karyotypes of four patients in this study (a: Case 1; b: case 2; c: Case 3; d: Case 4).
Case 2 involved a 25-year-old man who presented to the andrology department because of infertility after 2 years of marriage. Clinical examination showed normal intelligence and phenotype. Semen analysis revealed asthenospermia. Cytogenetic analysis revealed that his karyotype was 46,XY,inv(6)(p21q21) (Figure 1b), whereas that of his wife was 46,XX. Routine gynecological examination showed that his wife had a normal reproductive function.
Case 3 involved a 28-year-old man showing normal intelligence and phenotype who presented to the andrology department because his wife had two spontaneous miscarriages. Semen analysis revealed asthenospermia. Cytogenetic analysis revealed that his karyotype was 46,XY,inv(6)(p21.1q25) (Figure 1c) and that of his wife was 46,XX. Routine gynecological examination showed that his wife’s reproductive function was normal.
Case 4 involved a 33-year-old man who presented to the andrology department because of infertility after 5 years of marriage. He was diagnosed with azoospermia after undergoing semen analysis twice. Cytogenetic analysis revealed that his karyotype was 46,XY,inv(6)(p11q25) (Figure 1d), whereas that of his wife was 46,XX. Routine gynecological examination showed that his wife had a normal reproductive function.
3.2. Review of the literature
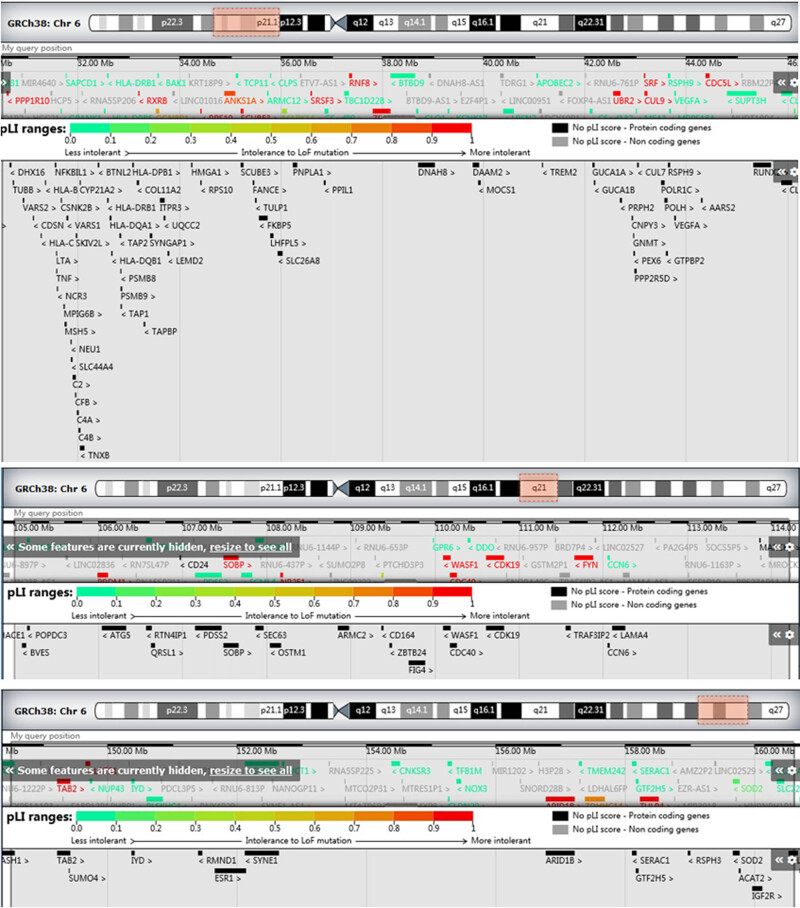
A total of ten carriers involving chromosomal 6 inversion were found in literature. Karyotype and clinical findings of these cases were collected and are summarized in Table 1. Including the four cases in our study, a total of seven cases of male infertility (including azoospermia, oligozoospermia, and asthenospermia) were identified. Four cases were associated with recurrent miscarriage, whereas the other three showed normal fertility. To analyze the possible causes of male infertility, we searched the related pathogenic genes at chromosomes 6p21, 6q21, and 6q25 using DECIPHER (https://www.deciphergenomics.org/; Figure 2). Meanwhile, genes associated with sperm function were searched using OMIM, and information on the genes and their loci and functions were collected. We identified six important genes at 6p21, 6q15, 6q21, and 6q25 that are related to spermatogenesis or sperm function (Table 2).
Table 1.
Clinical findings in the couples’ with a male partners carrying chromosome 6 inversion
| Cases | Karyotype | Family history | Clinical findings | Reference |
|---|---|---|---|---|
| 1 | inv(6)(p21q21) | N/A | Obstructed azoospermia | [25] |
| 2 | inv(6) (p23q25) | N/A | Recurrent miscarriage | [26] |
| 3 | inv(6)(p22q22) | N/A | Oligozoospermia | [27] |
| 4 | inv(6)(p23q23) | Pericentric inversions with observed recombinants in offspring | Normal fertility | [28] |
| 5 | inv(6)(p21q27) | Pericentric inversions with observed recombinants in offspring | Normal fertility | [28] |
| 6 | inv(6)(p22q24) | N/A | Recurrent abortion | [29] |
| 7 | inv(6)(p23q23.3) | N/A | Infertility | [30] |
| 8 | inv(6)(p21.3q25) | Daughter carrying inv(6)(p21.3q25) | Normal fertility | [31] |
| 9 | inv(6)(p22q24) | N/A | Recurrent miscarriage | [32] |
| 10 | inv(6)(p12q21) | N/A | Infertility | [33] |
| 11 | inv(6)(p23q13) | N/A | Recurrent miscarriage | This study |
| 12 | inv(6)(p21q21) | N/A | Asthenospermia | This study |
| 13 | inv(6)(p21.1q25) | N/A | Asthenospermia | This study |
| 14 | inv(6)(p11q25) | N/A | Azoospermia | This study |
N/A, not applicable.
Figure 2.
Genes associated with chromosomes 6p21, 6q21, and 6q25 in DECIPHER.
Table 2.
Breakpoints and genes related to sperm function on chromosome 6
| Breakpoint | Gene | Description/Phenotype | Gene function |
|---|---|---|---|
| 6p21 | SLC26A8 | Spermatogenic failure-3 | The mutations in the gene associated with severe asthenozoospermia |
| TCTE1 | T complex-associated testis-expressed 1 | TCTE1 knockout mice are sterile due to asthenozoospermia | |
| DNAH8 | Spermatogenic failure-46 | Spermatogenic failure-46 (SPGF46) is characterized by male infertility due to asthenoteratozoospermia | |
| 6q15 | SPACA1 | Sperm acrosome-associated protein 1 | SPACA1 is a transmembrane protein that localizes to the equatorial segment of spermatozoa and appears to function in sperm–egg fusion |
| 6q21 | ARMC2 | Spermatogenic failure-38 | Spermatogenic failure-38 is characterized by primary infertility and asthenoteratozoospermia due to multiple morphologic abnormalities of the flagella |
| 6q25 | ESR1 | Estrogen receptor 1 | ESRA/ESRB knockout mice were infertile. They exhibited various stages of spermatogenesis, but the numbers and motility of epididymal sperm were reduced significantly |
4. Discussion
Chromosomal inversion is a significant chromosomal structural abnormality. Male inversion carriers may be infertile because of spermatozoa production with unbalanced chromosome [17]. Another possible reason for male infertility is that DNA fragmentation is higher in sperm with unbalanced chromosomal content [18]. Although inversion carriers are potentially infertile, patients with normal fertility have often been observed in clinical practice. Therefore, genetic counseling remains a challenge for inversion carriers. This study reported four males showing pericentric inversion in chromosome 6. Two of these had asthenospermia, one had azoospermia, and the wife of the last patient had recurrent miscarriages.
Genetic counseling varies for inversion carriers showing different clinical phenotypes. For inversion carriers with recurrent spontaneous abortion, the couples can choose PGD to reduce the abortion rate and increase the chances of pregnancy [13]. Moreover, these carriers can opt for natural pregnancy along with prenatal diagnosis [10]. For inversion carriers with asthenospermia, PGD treatment should be recommended [19]. For inversion carriers with azoospermia, the possible etiology needs further analysis.
Through a literature search, the relationship between the breakpoint of chromosome 6 inversion and male infertility has been further reviewed. A total of seven patients showed infertility, azoospermia, oligozoospermia, or asthenospermia. The genomic region of the chromosome 6 is far too big. We searched the related pathogenic genes at chromosomes 6p21, 6q21, and 6q25 using DECIPHER, and found more than 60 important pathogenic genes on chromosome 6p21. To further explore the genes related to spermatogenesis, six important genes were identified at the breakpoint of chromosomal 6 inversion. The solute carrier family 26, member 8, T-complex-associated testes-expressed, and Dynein, axonemal, and heavy chain 8 genes are located on chromosome 6p21 and are associated with severe asthenozoospermia [20–22]. The carriers of inv(6)(p21q21) and inv(6)(p21.1q25) presented with asthenospermia, which may be related to the interference of these gene structures. The armadillo repeat-containing protein 2 gene mapped on chromosome 6 at 6q21 is associated with primary infertility and asthenoteratozoospermia [23]. The carrier of inv(6)(p12q21) (case 10 in Table 1) showed infertility, which may be related to the functional change of these genes. The estrogen receptor 1 gene located on chromosome 6q25 could influence spermatogenesis. ESRA/ESRB knockout mice were found to be infertile [24]. The carrier of inv(6)(p11q25) in this study showed azoospermia, which may be related to the gene change, and can be used for further analysis.
5. Conclusion
We report four male carriers of chromosome 6 inversion. Important genes associated with asthenospermia in chromosome 6p21 were found; these may be related to the clinical phenotype of these patients. Considered together with the published literature, these results suggest that physicians should focus on the breakpoints of inversion in genetic counseling.
Acknowledgments
We thank Liwen Bianji (Edanz) (www.liwenbianji.cn/), for editing the English text of a draft of this manuscript.
Footnotes
Funding information: No funds.
Conflict of interest: Authors state no conflict of interest.
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Babul-Hirji R, Hirji R, Chitayat D. Genetic counselling for infertile men of known and unknown etiology. Transl Androl Urol. 2021;10(3):1479–85. [DOI] [PMC free article] [PubMed]; Babul-Hirji R, Hirji R, Chitayat D. Genetic counselling for infertile men of known and unknown etiology. Transl Androl Urol. 2021;10(3):1479–85. doi: 10.21037/tau-2019-gcmi-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tang D, Lv M, Gao Y, Cheng H, Li K, Xu C, et al. Novel variants in helicase for meiosis 1 lead to male infertility due to non-obstructive azoospermia. Reprod Biol Endocrinol. 2021;19(1):129. [DOI] [PMC free article] [PubMed]; Tang D, Lv M, Gao Y, Cheng H, Li K, Xu C. et al. Novel variants in helicase for meiosis 1 lead to male infertility due to non-obstructive azoospermia. Reprod Biol Endocrinol. 2021;19(1):129. doi: 10.1186/s12958-021-00815-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Markandona O, Dafopoulos K, Anifandis G, Messini CI, Dimitraki M, Tsezou A, et al. Single-nucleotide polymorphism rs 175080 in the MLH3 gene and its relation to male infertility. J Assist Reprod Genet. 2015;32(12):1795–9. [DOI] [PMC free article] [PubMed]; Markandona O, Dafopoulos K, Anifandis G, Messini CI, Dimitraki M, Tsezou A. et al. Single-nucleotide polymorphism rs 175080 in the MLH3 gene and its relation to male infertility. J Assist Reprod Genet. 2015;32(12):1795–9. doi: 10.1007/s10815-015-0594-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Anifandis G, Markandona O, Dafopoulos K, Messini C, Tsezou A, Dimitraki M, et al. Embryological results of couples undergoing ICSI-ET treatments with males carrying the single nucleotide polymorphism rs175080 of the MLH3 gene. Int J Mol Sci. 2017;18(2):314. [DOI] [PMC free article] [PubMed]; Anifandis G, Markandona O, Dafopoulos K, Messini C, Tsezou A, Dimitraki M. et al. Embryological results of couples undergoing ICSI-ET treatments with males carrying the single nucleotide polymorphism rs175080 of the MLH3 gene. Int J Mol Sci. 2017;18(2):314. doi: 10.3390/ijms18020314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Singh P, Fragoza R, Blengini CS, Tran TN, Pannafino G, Al-Sweel N, et al. Human MLH1/3 variants causing aneuploidy, pregnancy loss, and premature reproductive aging. Nat Commun. 2021;12(1):5005. [DOI] [PMC free article] [PubMed]; Singh P, Fragoza R, Blengini CS, Tran TN, Pannafino G, Al-Sweel N. et al. Human MLH1/3 variants causing aneuploidy, pregnancy loss, and premature reproductive aging. Nat Commun. 2021;12(1):5005. doi: 10.1038/s41467-021-25028-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yammine T, Reynaud N, Lejeune H, Diguet F, Rollat-Farnier PA, Labalme A, et al. Deciphering balanced translocations in infertile males by next-generation sequencing to identify candidate genes for spermatogenesis disorders. Mol Hum Reprod. 2021;27(6):gaab034. [DOI] [PubMed]; Yammine T, Reynaud N, Lejeune H, Diguet F, Rollat-Farnier PA, Labalme A. et al. Deciphering balanced translocations in infertile males by next-generation sequencing to identify candidate genes for spermatogenesis disorders. Mol Hum Reprod. 2021;27(6):gaab034. doi: 10.1093/molehr/gaab034. [DOI] [PubMed] [Google Scholar]
- [7].Pelzman DL, Hwang K. Genetic testing for men with infertility: techniques and indications. Transl Androl Urol. 2021;10(3):1354–64. [DOI] [PMC free article] [PubMed]; Pelzman DL, Hwang K. Genetic testing for men with infertility: techniques and indications. Transl Androl Urol. 2021;10(3):1354–64. doi: 10.21037/tau-19-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Li R, Fan H, Zhang Q, Yang X, Zhan P, Feng S. Pericentric inversion in chromosome 1 and male infertility. Open Med (Wars). 2020;15(1):343–8. [DOI] [PMC free article] [PubMed]; Li R, Fan H, Zhang Q, Yang X, Zhan P, Feng S. Pericentric inversion in chromosome 1 and male infertility. Open Med (Wars) 2020;15(1):343–8. doi: 10.1515/med-2020-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sismani C, Rapti SM, Iliopoulou P, Spring A, Neroutsou R, Lagou M, et al. Novel pericentric inversion inv(9)(p23q22.3) in unrelated individuals with fertility problems in the Southeast European population. J Hum Genet. 2020;65(9):783–95. [DOI] [PubMed]; Sismani C, Rapti SM, Iliopoulou P, Spring A, Neroutsou R, Lagou M. et al. Novel pericentric inversion inv(9)(p23q22.3) in unrelated individuals with fertility problems in the Southeast European population. J Hum Genet. 2020;65(9):783–95. doi: 10.1038/s10038-020-0769-z. [DOI] [PubMed] [Google Scholar]
- [10].Zhang X, Shi Q, Liu Y, Jiang Y, Yang X, Liu R, et al. Fertility problems in males carrying an inversion of chromosome 10. Open Med (Wars). 2021;16(1):316–21. [DOI] [PMC free article] [PubMed]; Zhang X, Shi Q, Liu Y, Jiang Y, Yang X, Liu R. et al. Fertility problems in males carrying an inversion of chromosome 10. Open Med (Wars) 2021;16(1):316–21. doi: 10.1515/med-2021-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ghorbel M, Baklouti-Gargouri S, ElGhazel H, Zribi N, Ben Abdallah F, Cherif M, et al. Pericentric inversion of chromosome 12 [Inv (12) (p12q12)] associated with idiopathic azoospermia in one infertile Tunisian man. Biochem Biophys Res Commun. 2013;432(3):472–4. [DOI] [PubMed]; Ghorbel M, Baklouti-Gargouri S, ElGhazel H, Zribi N, Ben Abdallah F, Cherif M. et al. Pericentric inversion of chromosome 12 [Inv (12) (p12q12)] associated with idiopathic azoospermia in one infertile Tunisian man. Biochem Biophys Res Commun. 2013;432(3):472–4. doi: 10.1016/j.bbrc.2013.01.110. [DOI] [PubMed] [Google Scholar]
- [12].Morin SJ, Eccles J, Iturriaga A, Zimmerman RS. Translocations, inversions and other chromosome rearrangements. Fertil Steril. 2017;107(1):19–26. [DOI] [PubMed]; Morin SJ, Eccles J, Iturriaga A, Zimmerman RS. Translocations, inversions and other chromosome rearrangements. Fertil Steril. 2017;107(1):19–26. doi: 10.1016/j.fertnstert.2016.10.013. [DOI] [PubMed] [Google Scholar]
- [13].Bernicot I, Dechanet C, Mace A, Hedon B, Hamamah S, Pellestor F, et al. Predictive value of sperm-FISH analysis on the outcome of preimplantation genetic diagnosis (PGD) for a pericentric inversion inv5(p15.3q11.2) carrier. Hum Reprod. 2010;25(7):1818–23. [DOI] [PubMed]; Bernicot I, Dechanet C, Mace A, Hedon B, Hamamah S, Pellestor F. et al. Predictive value of sperm-FISH analysis on the outcome of preimplantation genetic diagnosis (PGD) for a pericentric inversion inv5(p15.3q11.2) carrier. Hum Reprod. 2010;25(7):1818–23. doi: 10.1093/humrep/deq101. [DOI] [PubMed] [Google Scholar]
- [14].Shao Y, Li J, Lu J, Li H, Zhu Y, Jiang W, et al. Clinical outcomes of Preimplantation genetic testing (PGT) application in couples with chromosomal inversion, a study in the Chinese Han population. Reprod Biol Endocrinol. 2020;18(1):79. [DOI] [PMC free article] [PubMed]; Shao Y, Li J, Lu J, Li H, Zhu Y, Jiang W. et al. Clinical outcomes of Preimplantation genetic testing (PGT) application in couples with chromosomal inversion, a study in the Chinese Han population. Reprod Biol Endocrinol. 2020;18(1):79. doi: 10.1186/s12958-020-00635-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dutta UR, Hansmann I, Schlote D. Molecular cytogenetic characterization of a familial pericentric inversion 3 associated with short stature. Eur J Med Genet. 2015;58(3):154–9. [DOI] [PubMed]; Dutta UR, Hansmann I, Schlote D. Molecular cytogenetic characterization of a familial pericentric inversion 3 associated with short stature. Eur J Med Genet. 2015;58(3):154–9. doi: 10.1016/j.ejmg.2015.01.001. [DOI] [PubMed] [Google Scholar]
- [16].Ciuladaite Z, Preiksaitiene E, Utkus A, Kučinskas V. Relatives with opposite chromosome constitutions, rec(10)dup(10p)inv(10)(p15.1q26.12) and rec(10)dup(10q)inv(10)(p15.1 q26.12), due to a familial pericentric inversion. Cytogenet Genome Res. 2014;144(2):109–13. [DOI] [PubMed]; Ciuladaite Z, Preiksaitiene E, Utkus A, Kučinskas V. Relatives with opposite chromosome constitutions, rec(10)dup(10p)inv(10)(p15.1q26.12) and rec(10)dup(10q)inv(10)(p15.1 q26.12), due to a familial pericentric inversion. Cytogenet Genome Res. 2014;144(2):109–13. doi: 10.1159/000368863. [DOI] [PubMed] [Google Scholar]
- [17].Caer E, Perrin A, Douet-Guilbert N, Amice V, De Braekeleer M, Morel F. Differing mechanisms of meiotic segregation in spermatozoa from three carriers of a pericentric inversion of chromosome 8. Fertil Steril. 2008;89(6):1637–40. [DOI] [PubMed]; Caer E, Perrin A, Douet-Guilbert N, Amice V, De Braekeleer M, Morel F. Differing mechanisms of meiotic segregation in spermatozoa from three carriers of a pericentric inversion of chromosome 8. Fertil Steril. 2008;89(6):1637–40. doi: 10.1016/j.fertnstert.2007.04.056. [DOI] [PubMed] [Google Scholar]
- [18].Perrin A, Nguyen MH, Bujan L, Vialard F, Amice V, Guéganic N, et al. DNA fragmentation is higher in spermatozoa with chromosomally unbalanced content in men with a structural chromosomal rearrangement. Andrology. 2013;1(4):632–8. [DOI] [PubMed]; Perrin A, Nguyen MH, Bujan L, Vialard F, Amice V, Guéganic N. et al. DNA fragmentation is higher in spermatozoa with chromosomally unbalanced content in men with a structural chromosomal rearrangement. Andrology. 2013;1(4):632–8. doi: 10.1111/j.2047-2927.2013.00100.x. [DOI] [PubMed] [Google Scholar]
- [19].Won SY, Kim H, Lee WS, Kim JW, Shim SH. Pre-implantation genetic diagnosis and pre-implantation genetic screening: two years experience at a single center. Obstet Gynecol Sci. 2018;61(1):95–101. [DOI] [PMC free article] [PubMed]; Won SY, Kim H, Lee WS, Kim JW, Shim SH. Pre-implantation genetic diagnosis and pre-implantation genetic screening: two years experience at a single center. Obstet Gynecol Sci. 2018;61(1):95–101. doi: 10.5468/ogs.2018.61.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rode B, Dirami T, Bakouh N, Rizk-Rabin M, Norez C, Lhuillier P, et al. The testis anion transporter TAT1 (SLC26A8) physically and functionally interacts with the cystic fibrosis transmembrane conductance regulator channel: a potential role during sperm capacitation. Hum Mol Genet. 2012;21(6):1287–98. [DOI] [PubMed]; Rode B, Dirami T, Bakouh N, Rizk-Rabin M, Norez C, Lhuillier P. et al. The testis anion transporter TAT1 (SLC26A8) physically and functionally interacts with the cystic fibrosis transmembrane conductance regulator channel: a potential role during sperm capacitation. Hum Mol Genet. 2012;21(6):1287–98. doi: 10.1093/hmg/ddr558. [DOI] [PubMed] [Google Scholar]
- [21].Castaneda JM, Hua R, Miyata H, Oji A, Guo Y, Cheng Y, et al. TCTE1 is a conserved component of the dynein regulatory complex and is required for motility and metabolism in mouse spermatozoa. Proc Natl Acad Sci USA. 2017;114(27):E5370–8. [DOI] [PMC free article] [PubMed]; Castaneda JM, Hua R, Miyata H, Oji A, Guo Y, Cheng Y. et al. TCTE1 is a conserved component of the dynein regulatory complex and is required for motility and metabolism in mouse spermatozoa. Proc Natl Acad Sci USA. 2017;114(27):E5370–8. doi: 10.1073/pnas.1621279114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yang Y, Jiang C, Zhang X, Liu X, Li J, Qiao X, et al. Loss-of-function mutation in DNAH8 induces asthenoteratospermia associated with multiple morphological abnormalities of the sperm flagella. Clin Genet. 2020;98(4):396–401. [DOI] [PubMed]; Yang Y, Jiang C, Zhang X, Liu X, Li J, Qiao X. et al. Loss-of-function mutation in DNAH8 induces asthenoteratospermia associated with multiple morphological abnormalities of the sperm flagella. Clin Genet. 2020;98(4):396–401. doi: 10.1111/cge.13815. [DOI] [PubMed] [Google Scholar]
- [23].Coutton C, Martinez G, Kherraf ZE, Amiri-Yekta A, Boguenet M, Saut A, et al. Bi-allelic mutations in ARMC2 lead to severe astheno-teratozoospermia due to sperm flagellum malformations in humans and mice. Am J Hum Genet. 2019;104(2):331–40. [DOI] [PMC free article] [PubMed]; Coutton C, Martinez G, Kherraf ZE, Amiri-Yekta A, Boguenet M, Saut A. et al. Bi-allelic mutations in ARMC2 lead to severe astheno-teratozoospermia due to sperm flagellum malformations in humans and mice. Am J Hum Genet. 2019;104(2):331–40. doi: 10.1016/j.ajhg.2018.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Couse JF, Hewitt SC, Bunch DO, Sar M, Walker VR, Davis BJ, et al. Postnatal sex reversal of the ovaries in mice lacking estrogen receptors alpha and beta. Science. 1999;286(5448):2328–31. [DOI] [PubMed]; Couse JF, Hewitt SC, Bunch DO, Sar M, Walker VR, Davis BJ. et al. Postnatal sex reversal of the ovaries in mice lacking estrogen receptors alpha and beta. Science. 1999;286(5448):2328–31. doi: 10.1126/science.286.5448.2328. [DOI] [PubMed] [Google Scholar]
- [25].Matsuda T, Horii Y, Ogura K, Nonomura M, Okada K, Yoshida O. Chromosomal survey of 1001 subfertile males: incidence and clinical features of males with chromosomal anomalies. Hinyokika Kiyo. 1992;38(7):803–9. [PubMed]; Matsuda T, Horii Y, Ogura K, Nonomura M, Okada K, Yoshida O. Chromosomal survey of 1001 subfertile males: incidence and clinical features of males with chromosomal anomalies. Hinyokika Kiyo. 1992;38(7):803–9. [PubMed] [Google Scholar]
- [26].Anton E, Blanco J, Egozcue J, Vidal F. Risk assessment and segregation analysis in a pericentric inversion inv6p23q25 carrier using FISH on decondensed sperm nuclei. Cytogenet Genome Res. 2002;97(3–4):149–54. [DOI] [PubMed]; Anton E, Blanco J, Egozcue J, Vidal F. Risk assessment and segregation analysis in a pericentric inversion inv6p23q25 carrier using FISH on decondensed sperm nuclei. Cytogenet Genome Res. 2002;97(3–4):149–54. doi: 10.1159/000066603. [DOI] [PubMed] [Google Scholar]
- [27].Gabriel-Robez O, Ratomponirina C, Croquette M, Maetz JL, Couturier J, Rumpler Y. Reproductive failure and pericentric inversion in man. Andrologia. 1987;19(6):662–9. [DOI] [PubMed]; Gabriel-Robez O, Ratomponirina C, Croquette M, Maetz JL, Couturier J, Rumpler Y. Reproductive failure and pericentric inversion in man. Andrologia. 1987;19(6):662–9. doi: 10.1111/j.1439-0272.1987.tb01924.x. [DOI] [PubMed] [Google Scholar]
- [28].Kaiser P. Pericentric inversions. Problems and significance for clinical genetics. Hum Genet. 1984;68(1):1–47. [DOI] [PubMed]; Kaiser P. Pericentric inversions. Problems and significance for clinical genetics. Hum Genet. 1984;68(1):1–47. doi: 10.1007/BF00293869. [DOI] [PubMed] [Google Scholar]
- [29].Portnoï MF, Joye N, van den Akker J, Morlier G, Taillemite JL. Karyotypes of 1142 couples with recurrent abortion. Obstet Gynecol. 1988;72(1):31–4. [PubMed]; Portnoï MF, Joye N, van den Akker J, Morlier G, Taillemite JL. Karyotypes of 1142 couples with recurrent abortion. Obstet Gynecol. 1988;72(1):31–4. [PubMed] [Google Scholar]
- [30].Young D, Klepacka D, McGarvey M, Schoolcraft WB, Katz-Jaffe MG. Infertility patients with chromosome inversions are not susceptible to an inter-chromosomal effect. J Assist Reprod Genet. 2019;36(3):509–16. [DOI] [PMC free article] [PubMed]; Young D, Klepacka D, McGarvey M, Schoolcraft WB, Katz-Jaffe MG. Infertility patients with chromosome inversions are not susceptible to an inter-chromosomal effect. J Assist Reprod Genet. 2019;36(3):509–16. doi: 10.1007/s10815-018-1376-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sanyal D, Bhairi V, Kadandale JS. Practice of consanguinity and unusual cases of inherited familial chromosome abnormalities: a case report. Int J Mol Cell Med. 2016;5(1):57–63. [PMC free article] [PubMed]; Sanyal D, Bhairi V, Kadandale JS. Practice of consanguinity and unusual cases of inherited familial chromosome abnormalities: a case report. Int J Mol Cell Med. 2016;5(1):57–63. [PMC free article] [PubMed] [Google Scholar]
- [32].Pan CS, Qiu XF, Huang XX, Weng ZL, Huang XF. FISH analysis of meiotic segregation results of the spermatozoa from male pericentric inversion carriers. Zhonghua Nan Ke Xue. 2012;18(4):344–8. [PubMed]; Pan CS, Qiu XF, Huang XX, Weng ZL, Huang XF. FISH analysis of meiotic segregation results of the spermatozoa from male pericentric inversion carriers. Zhonghua Nan Ke Xue. 2012;18(4):344–8. [PubMed] [Google Scholar]
- [33].Black LD, Nudell DM, Cha I, Cherry AM, Turek PJ. Compound genetic factors as a cause of male infertility: case report. Hum Reprod. 2000;15(2):449–51. [DOI] [PubMed]; Black LD, Nudell DM, Cha I, Cherry AM, Turek PJ. Compound genetic factors as a cause of male infertility: case report. Hum Reprod. 2000;15(2):449–51. doi: 10.1093/humrep/15.2.449. [DOI] [PubMed] [Google Scholar]