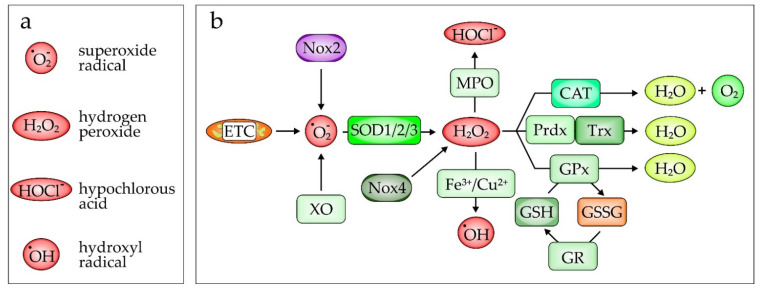
Figure 2.
Reactive oxygen species (ROS) and ROS-generating and -scavenging enzymes. (a) ROS involved in ARDS. (b) The superoxide radical O2− is generated by the NADPH oxidase 2 (Nox2), the xanthine oxidase (XO), and the electron transfer chain (ETC) located in the mitochondria. O2− is dismutated to hydrogen peroxide (H2O2) by one of three superoxide dismutases (SODs), which are located in the cytosol (SOD1 ≙ CuZnSOD), in mitochondria (SOD2 ≙ MnSOD), or extracellularly, often associated with the extracellular matrix (SOD3 ≙ EC-SOD). One further source of H2O2 is Nox4, which is located in mitochondria or endoplasmic reticulum (ER) of endothelial as well as epithelial cells. H2O2 is the substrate for the myeloperoxidase-(MPO)-derived oxidant hypochlorous acid (HOCl−), known to cause tissue injury. Stored in neutrophil granules, MPO is released following neutrophil activation. In the Fenton reaction, H2O2 is further metabolized to the highly antimicrobial hydroxyl radical (˙OH). ROS-scavenging enzymes, such as catalase (CAT) or glutathione peroxidase (GPx), detoxify H2O2 to H2O and O2. To achieve this, GPx oxidizes GSH to GSSG, which in return is reduced via the glutathione reductase to GSH. Similarly, peroxiredoxin (Prx), belonging to a small family of peroxidases, reduces H2O2 by oxidizing thioredoxin (Trx), which then is restored to the reduced form by the thioredoxin reductase (not shown).