Figure 3.
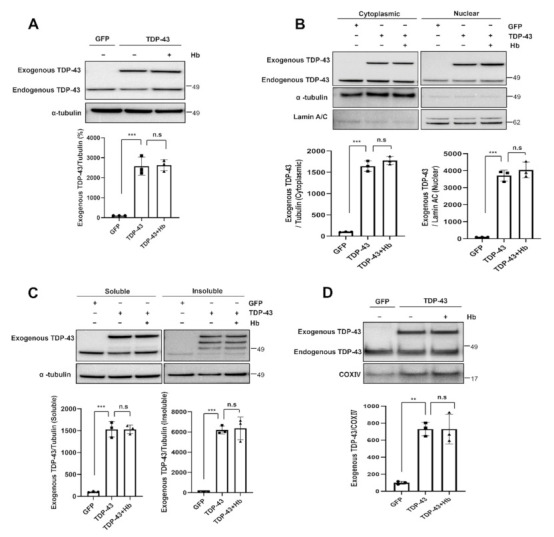
Hydroxocobalamin did not affect the aggregation and mislocalization of TDP-43. In the immunoblot analysis, cells were infected with the GFP- or GFP-tagged human WT TDP-43 lentivirus and grown for 2 days. The day before harvest, cells were treated with Hb (10 µM) for 24 h. (A) Cell lysates were prepared, and samples were immunoblotted with anti-TDP-43 antibodies. Hb treatment did not affect the TDP-43 protein levels. (B) Cytoplasmic and nuclear localization of exogenous TDP-43 in neuronal cells treated with Hb. Bar diagrams showing cytoplasmic and nuclear TDP-43 levels normalized to α-tubulin or Lamin A/C, respectively, are shown. Data are presented as the means ± SD of three independent experiments. *** p < 0.001 and n.s., not significant (one-way ANOVA with Tukey’s multiple comparison test). (C) Immunoblot analysis of soluble and insoluble exogenous TDP-43 protein levels in TDP-43-expressing cells treated with Hb. Error bars represent the mean values ± SD of three independent experiments. *** p < 0.001 and n.s., not significant (one-way ANOVA with Tukey’s multiple comparison test). (D) Immunoblot analysis showing the localization of the exogenous TDP-43 protein in the mitochondrial fraction. Normalization was performed using COX IV as a marker of purified mitochondria. Data are presented as the means ± SD of three independent experiments. Statistical analyses were performed using one-way ANOVA followed by Tukey’s multiple comparison test. ** p < 0.01, *** p < 0.001, and n.s., not significant.
