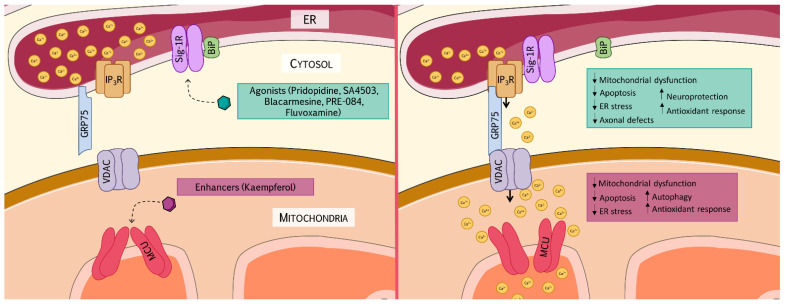
Figure 1.
Schematic representation showing the effects of mitochondrial Ca2+ uptake promoters. The ER is the main Ca2+ storage in the cell and Ca2+ exchange between the ER and the mitochondria requires the formation of tethers composed by proteins of both compartments. Sig-1R resides in the ER membrane, in a dormant, Ca2+-dependent state. Upon activation by agonists, Sig-1R dissociates from BiP/GRP78 and reallocates within the ER membrane, interacting with IP3R and chaperoning the protein complex that transfers Ca2+ to the mitochondria. This complex formed by IP3R-GRP75-VDAC ensures a rapid Ca2+ flux to the mitochondrial intermembrane space, which triggers MCU opening and Ca2+ to cross the mitochondrial inner membrane. Several models of neurological disorders such as ALS, CMT and FRDA have exhibited alterations in mitochondrial Ca2+ buffering by defective appositions between the two organelles. Both Sig-1R agonists and MCU enhancers promote Ca2+ exchange between the ER and the mitochondria, exerting beneficial effects in different models of neurological diseases. On the one hand, Sig-1R agonists (pridopidine, SA4503, Blacarmesine, PRE-084, and fluvoxamine) have been demonstrated to exert neuroprotective effects, improving mitochondrial dysfunction, preventing cells from apoptosis, activating the antioxidant response, ameliorating ER stress, and improving axonal defects. On the other hand, the MCU enhancer, Kaempferol, has helped to improve mitochondrial dysfunction, activate the oxidative stress response, modulate autophagy, regulate ER stress, and prevent cells from apoptosis. This figure has been created using Creative Commons resources from Servier Medical Art [99]. ALS: amyotrophic lateral sclerosis; Bip/GRP78: binding immunoglobulin protein/glucose-regulated protein 78; CMT: Charcot–Marie–Tooth; ER: endoplasmic reticulum; FRDA: Friedreich’s ataxia; GRP75: glucose-regulated protein 75; IP3R: inositol 1,4,5-trisphosphate receptor; MCU: mitochondrial calcium uniporter; Sig-1R: sigma non-opioid intracellular receptor 1; VDAC: voltage-dependent anion channel.