Abstract
Hip fractures are associated with significant morbidity and mortality in smokers with lung disease, but whether lung-specific factors are associated with fracture risk is unknown. Our goal was to determine whether lung-specific factors associate with incident hip fracture and improve risk discrimination of traditional fracture risk models in smokers. The analysis consisted of a convenience sample of 9,187 current and former smokers (58,477 participant follow-up years) participating in the Genetic Epidemiology of COPD longitudinal observational cohort study. Participants were enrolled between 2008–2011 with follow-up data collection through July 2018. Traditional risk factors associated with incident hip fracture (n=361) included age, female sex, osteoporosis, prevalent spine and hip fracture, rheumatoid arthritis, and diabetes. Lung-specific risk factors included post-bronchodilator FEV1% predicted (OR 0.95, 95% CI 0.92–0.99 for each 10% increase), GOLD classification (OR 1.09, 95% CI 1.002–1.19 for each higher stage), presence of CT-determined emphysema (OR 1.34, 95% CI 1.06–1.69), symptom scores (OR 1.10, 95% CI 1.03–1.19 for each higher unit score), six-minute walk distance (OR 0.92, 95% CI 0.90–0.95 for each 30 meter increase), BODE index (OR 1.07, 95% CI 1.01–1.13 for each higher unit score), total exacerbations (OR 1.13, 95% CI 1.10–1.16 per exacerbation), and annual exacerbations (OR 1.37, 95% CI 1.21–1.55 per exacerbation). In multivariable modeling, age, African American race, osteoporosis, prevalent hip and spine fracture, rheumatoid arthritis, and diabetes were associated with incident hip fracture. The presence of emphysema, six-minute walk distance, and total number of exacerbations added to traditional models improved risk discrimination (integrated discrimination improvement values 0.001 (95% CI 0.0003–0.002), 0.001 (95% CI 0.0001–0.002), and 0.008 (95% CI 0.003–0.013) corresponding to a relative IDI of 12.8%, 6.3%, and 34.6%). These findings suggest that the incorporation of lung-specific risk factors into fracture risk assessment tools may more accurately predict fracture risk in smokers.
Keywords: Fracture risk assessment, osteoporosis, screening, pulmonary disease, chronic obstructive, smoking
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death worldwide, affecting approximately 10% of adults.(1) COPD-related comorbidities substantially contribute to disease burden.(2) Low bone mineral density (BMD) is common in smokers with lung disease,(3, 4) often in the absence of defined risk factors.(5, 6) Osteoporotic fractures are likewise prevalent and associated with worse outcomes in COPD patients.(3, 7) Efforts aimed at primary prevention of fractures in individuals with COPD are hindered by ambiguity regarding osteoporosis risk attributable to chronic lung disease in established osteoporosis screening guidelines (8, 9) and lack of defined osteoporosis screening strategies in COPD management clinical guidelines. As such, physicians are not routinely ordering BMD assessments in current and former smokers with lung disease, resulting in underdiagnosis of osteoporosis in COPD.(10)
Studies have demonstrated an independent association between low BMD and both CT-determined emphysema (11–14) and acute exacerbations of COPD.(15) Whether these lung disease-specific risk factors for low BMD are relevant to osteoporotic fracture risk is unknown given that BMD is a surrogate predictor of actual fracture risk. Previous studies showing an association between smoking and fracture risk lack the detailed lung phenotyping necessary to adequately study lung-disease specific risks.(16, 17) Understanding the impact of these lung specific factors may provide screening guidance to clinicians caring for heavy smokers and COPD patients and provide data for future guideline development.
The Genetic Epidemiology of COPD (COPDGene) study was designed to examine genetic determinants of COPD and COPD progression.(18) Entering its tenth year of data collection, the COPDGene cohort provides longitudinal clinical events, physiologic, and radiographic data in a large cohort of smokers with and without airflow obstruction. Our objective was to leverage the wealth of baseline and longitudinal data available in the COPDGene cohort to examine risk factors in smokers associated with incident hip fracture with the ultimate goal of identifying lung disease-related risk factors that may inform decisions regarding early osteoporosis screening in select smokers with COPD. We hypothesized that both established and lung-disease specific factors associated with low BMD in prior studies would be associated with incident hip fracture in current and former smokers. We further hypothesized that these lung-disease specific factors would significantly improve risk discrimination for incident hip fracture when added to traditional risk factor models.
MATERIALS AND METHODS
Study Population
The study population consisted of participants enrolled in the Genetic Epidemiology of COPD (COPDGene) study who participated in longitudinal follow-up with at least one follow-up contact. COPDGene is a multi-center, observational, longitudinal cohort study designed to examine genetic factors associated with COPD. Baseline enrollment occurred between 2008–2011. Follow-up data collected through July 2018 was included in the analysis. Participants were recruited from the communities of the 21 study centers. Inclusion criteria included individuals age 45–80 who self-identify as either non-Hispanic Caucasian or non-Hispanic African American with a minimum of ten pack-years tobacco exposure. Current and former smokers both with and without COPD, defined as a forced expiratory volume in the 1st second (FEV1) to forced vital capacity (FVC) ratio < 0.70 on post-bronchodilator spirometry, were enrolled. Individuals with interstitial lung disease, other significant lung disease or a history of lung transplantation were excluded. Detailed inclusion and exclusion criteria have been previously described.(18) A convenience sample that included all COPDGene participants with a baseline study visit and at least one longitudinal follow-up contact was included in the analysis. Institutional Review Board approval was obtained at each center and written, informed consent was obtained for each participant.
Study Measurements
Baseline study procedures included demographic and clinical questionnaires, symptom questionnaires [modified Medical Research Council Questionnaire (mMRC), ranging from 0 to 4], pre- and post-bronchodilator spirometry, a chest computed tomography (CT) scan, and a six-minute walk test. Spirometry was performed before and ten minutes after two puffs of albuterol in accordance with ATS guidelines.(19) CT scans were obtained during a breath hold at coached total lung capacity and at functional residual capacity or residual volume by either 16- or 64-detector CT scanners. Images were obtained at 120 kV tube voltage and 200 mA tube current and reconstructed using a high-resolution reconstruction algorithm with 1-mm slice thickness and 10-mm reconstruction intervals. Percent emphysema was defined as the percentage of lung tissue voxels with attenuation values <−950 Hounsfield Units (HU) on inspiratory scan and determined using VIDA (VIDA Diagnostics, Inc) quantitative image analysis software.(20) Emphysema presence was defined by greater than 5% of lung volume with CT attenuation values less than −950 HU (≥ 5% emphysema).
Longitudinal Follow-Up
Longitudinal follow-up of participants began in May 2009. Participants were contacted at 3- to 6-month intervals via automated telephone calls, web-based surveys, or coordinator phone calls. During follow-up encounters, participants were asked about hospitalizations, smoking habits, respiratory symptoms, the incidence, frequency, and severity of COPD exacerbations, and the incidence of new medical conditions that occurred since the time of previous contact. Participants were also asked about new hip fractures that occurred during the follow-up interval. Vital status was assessed through follow-up tracking of subjects, secondary contacts and searching the social security death index. Follow-up data through July 2018 for both living and deceased participants were included in the final analysis.
Statistical Analysis
Differences in demographic, clinical, and radiographic characteristics, lung function, medication use, exacerbation frequency during follow-up, and pre-existing comorbidities between participants with and without incident hip fracture were compared using Welch T test for continuous variables and the Chi-Square test for categorical variables. Lung function was reported as both a continuous (FEV1%) and categorical variable. Categories of lung function included: GOLD 0 – current and former smokers with normal spirometry (FEV1/FVC ≥0.70 and FEV1% and FVC% both ≥ 80% predicted); GOLD 1 – FEV1/forced vital capacity (FVC) ≤ 70, FEV1% ≥ 80; GOLD 2 – FEV1/FVC < 70, 50 ≤ FEV1% < 80; GOLD 3 – FEV1/FVC < 70, 30 ≤ FEV1% < 50; GOLD 4 – FEV1/FVC < 70, FEV1% < 30. Unclassified or preserved ratio impaired spirometry (PRIsM) was defined as an FEV1/FVC ratio ≥ 70 but FEV1 < 80% predicted. CT-determined emphysema was reported as both a continuous and dichotomous variable with emphysema presence defined by a quantitative threshold of ≥ 5%, the minimum percentage needed in a general population for emphysema to be considered present.(21) Treatment with bone-modifying therapy was defined by self-reported use of bisphosphonates or hormonal therapy including estrogen, testosterone, or parathyroid hormone. Prevalent osteoporosis, hip and spine fracture, rheumatoid arthritis, and diabetes were based on self-report. As part of a separate study, a subset of participants (n=2830) were assessed for vertebral fracture on chest CT scan using previously described methods.(3) Severe exacerbations were defined as acute exacerbations requiring hospitalization. The analyses were performed in participants with available follow-up data, which included 9187 out of 10612 recruited (13.4% lost to follow up). Final models included 8994 subjects. Univariable and multivariable analyses were performed with available-case analysis (pairwise) and complete-case analysis (listwise), respectively.
The primary outcome of interest was self-reported incident hip fracture during follow-up. Established osteoporosis risk factors, including age, sex, race, current smoking, oral steroid use, prevalent self-reported osteoporosis, hip or spine fracture, rheumatoid arthritis, and diabetes, and potential lung disease-specific osteoporosis risk factors, including obstruction severity, emphysema, inhaled corticosteroids, six-minute walk distance, body mass index (BMI), respiratory symptoms, and acute exacerbation frequency, were the primary exposures of interest. The association of established risk factors with incident hip fracture was assessed with univariable and multivariate logistic regression analyses. Predictive models were developed using traditional risk factors for osteoporosis and three separate modeling approaches (backward selection, Bayesian information criterion [BIC],(22) and Bayesian Model Averaging [BMA]). In the BMA approach, the best fitting model that included variables with posterior effect probability (PEP)>0.5 was reported. Factors established in the literature as risk factors for fracture, regardless of their significance level in univariable analysis in our cohort, were included in the final model. A sensitivity analysis was performed in the subset of participants with CT-assessed vertebral compression fractures (n=2830) in which CT-assessed vertebral fracture was substituted for self-reported vertebral compression fracture in the predictive modeling. The association of lung disease-specific exposures with incident hip fracture was assessed individually with logistic regression models adjusted for established osteoporosis risk factors.
To determine the added value of including lung disease-specific risk factors in a fracture prediction model, lung disease-specific risk factors were first individually added to the established osteoporosis risk factor model with the greatest number of significant established clinical predictors using the three modeling approaches and the net reclassification improvement (NRI), the integrated discrimination improvement (IDI), and relative IDI were calculated.(23) NRI and IDI capture the marginal strength of a new predictor, in this case a lung-disease specific fracture risk factor, while accounting for correlations with variables included in the baseline model.(23) NRI in patients with (event) and without (non-event) new hip fracture were reported separately for each lung disease-specific risk marker using a threshold of 3% hip fracture risk for each model.(24) Adjusted odds ratio (aOR) and change in likelihood ratio (from traditional model) for each lung-specific predictor were likewise calculated.
Our goal was to determine the model with the highest predictive performance regardless of whether the model’s predictors were independently associated with incident hip fracture in our univariable logistic analyses. We combined both traditional and lung-specific risk factors in a single model using three modeling approaches (backwards selection, BIC, and BMA) and selected the model with the best discrimination power (bootstrapped AUC) and calibration (Hosmer-Lemeshow goodness of fit). Statistical analyses were performed with Stata 15.1 (StataCorp, College Station, TX) and R 3.6.1 (Vienna, Austria) BMA and StepAIC packages.
RESULTS
Subject Characteristics
A total of 9,187 participants were followed longitudinally over a median 7.4 years (total of 58,477 participant follow-up years). Participants were 51.4% male with a median age of 60 years (Table 1). Tobacco burden was high with nearly half of the participants actively smoking at study entry. At enrollment less than half of the participants had evidence of airflow obstruction and only one-third had emphysema present on chest CT. The presence of major risk factors, including reported prevalent fracture, systemic steroid use, and rheumatoid arthritis, was low at 6.5%, 2.5%, and 7.3% respectively. The prevalence of diabetes, which is associated with an increased fracture risk, was 13.2%. The self-reported prevalence of osteoporosis was 9.6% while only 6% of the cohort reported the use of bone-modifying therapy, and the reported prevalence of vertebral compression and hip fractures was 4.7% and 1.8%, respectively (Table 1). Approximately 33% had some evidence of vertebral fracture on chest CT scan.
Table 1:
Baseline Participant Characteristics
| ALL N=9187 |
NO HIP FRACTURE N=8826 |
HIP FRACTURE N=361 |
P VALUE | |
|---|---|---|---|---|
| Age, median (IQR) | 60.0 (52.7–67.2) | 59.9 (52.7–67.1) | 63.1 (55.3–70.6) | <0.001 |
| Male gender, n (%) | 4719 (51.4) | 4552 (51.6) | 167 (46.3) | 0.048 |
| Race | ||||
| Caucasian, n (%) | 6530 (71.1) | 6290 (71.3) | 240 (66.5) | 0.049 |
| African American, n (%) | 2657 (28.9) | 2536 (28.7) | 121 (33.5) | |
| BMIa, median (IQR) | 28.1 (24.5–32.4) | 28.1 (24.5–32.4) | 28.2 (24.2–33.5) | 0.52 |
| Current smoker, n (%) | 4309 (48.5) | 4140 (48.5) | 169 (47.1) | 0.59 |
| Pack years, median (IQR) | 39.5 (27.7–55.5) | 39.5 (27.8–55.3) | 40.4 (26.3–57.0) | 0.33 |
| FEV1b % predicted post, median (IQR) | 80.0 (58.7–94.5) | 80.2 (58.9–94.6) | 75.0 (54.0–92.6) | 0.024 |
| FEV1/FVCc post ratio, median (IQR) | 0.71 (0.57–0.78) | 0.71 (0.57–0.78) | 0.71 (0.57–0.78) | 0.14 |
| GOLD, n (%) | 0.009 | |||
| 0 | 3737 (42.3) | 3613 (42.6) | 124 (34.7) | |
| 1 | 693 (7.8) | 667 (7.9) | 26 (7.3) | |
| 2 | 1722 (19.5) | 1648 (19.4) | 74 (20.7) | |
| 3 | 1069 (12.1) | 1014 (12.0) | 55 (15.4) | |
| 4 | 525 (5.9) | 508 (6.0) | 17 (4.8) | |
| Unclassified PRiSMd | 1094 (12.4) | 1033 (12.2) | 61 (17.1) | |
| Percent emphysema, median (IQR) | 2.2 (0.7–7.4) | 2.2 (0.7–7.3) | 3.1 (0.9–8.9) | 0.14 |
| Percent emphysema ≥ 5%, n (%) | 2513 (32.4) | 2396 (32.1) | 117 (38.7) | 0.016 |
| Inhaled corticosteroid use, n (%) | 2202 (24.8) | 2098 (24.6) | 104 (29.2) | 0.05 |
| Oral corticosteroid use, n (%) | 219 (2.5) | 206 (2.5) | 13 (3.7) | 0.14 |
| Prior oral or parenteral steroids, n (%) | 1482 (16.6) | 1418 (16.5) | 64 (17.8) | 0.52 |
| Reported osteoporosis, n (%) | 849 (9.6) | 784 (9.2) | 65 (18.1) | <0.001 |
| Treated with bone-modifying agent | 555 (6.0) | 522 (5.9) | 33 (9.4) | 0.012 |
| Prior hip fracture, n (%) | 161 (1.8) | 130 (1.5) | 31 (8.6) | <0.001 |
| Reported vertebral fracture, n (%) | 413 (4.7) | 377 (4.4) | 36 (10.0) | <0.001 |
| CT assessed vertebral fracture* | 920 (32.5) | 879 (32.2) | 41 (41.0) | 0.065 |
| Reported rheumatoid arthritis, n (%) | 647 (7.3) | 600 (7.0) | 47 (13.1) | <0.001 |
| Reported diabetes, n (%) | 1172 (13.2) | 1099 (12.9) | 73 (20.3) | <0.001 |
| Six-minute walk distance (meters) | 422 (342–495) | 425 (344–497) | 381 (296–457) | <0.001 |
| mMRCe, mean (SD) | 1.3 (1.4) | 1.3 (1.4) | 1.5 (1.4) | 0.007 |
| Total exacerbations, mean (SD) | 2.3 (4.7) | 2.2 (4.6) | 4.2 (6.1) | <0.001 |
| Total Severe exacerbations, mean (SD) | 0.8 (2.1) | 0.7 (2.0) | 1.8 (3.8) | <0.001 |
| Exacerbations/year, mean (SD) | 0.4 (0.9) | 0.4 (0.9) | 0.6 (1.0) | <0.001 |
| Severe Exacerbations/year, mean (SD) | 0.1 (0.5) | 0.1 (0.5) | 0.3 (0.6) | <0.001 |
| Died, n (%) | 1429 (15.6) | 1361 (15.4) | 68 (18.8) | 0.079 |
Body mass index
Forced expiratory volume
Forced vital capacity
Preserved ratio, impaired spirometry
Modified medical research council symptom questionnaire
N = 2830
Factors Associated with Incident Hip Fracture
During follow-up, there were 361 incident hip fractures reported. Participants with incident hip fractures were older, were more likely to be female or African American, had lower FEV1% predicted, greater inhaled steroid use, greater emphysema, greater symptoms, shorter six-minute walk distances, and higher reported pre-existing fractures and comorbidities (Table 1). The total number and annual rate of acute exacerbations and exacerbations requiring hospitalization were increased in participants experiencing incident hip fracture during follow-up. There was no statistically significant difference in oral steroid use or prior treatment with oral or parenteral steroids between groups. A trend toward increased mortality occurred in the incident hip fracture group (19% vs 15%, p=0.08).
Among established osteoporosis risk factors, older age (OR 1.03, 95% CI 1.02–1.05 per year), female sex (OR 1.23, 95% CI 1.0–1.54), personal history of osteoporosis (OR 2.18, 95% CI 1.65–2.89), prevalent self-reported compression fracture (OR 2.41, 95% CI 1.68–3.45), prevalent self-reported hip fracture (OR 6.11, 95% CI 4.06–9.17), rheumatoid arthritis (OR 1.99, 95% CI 1.45–2.74), and diabetes (OR 1.73, 95% CI 1.32–2.25) were associated with increased hip fracture risk. BMI, smoking status, CT-assessed prevalent vertebral fracture, and baseline oral steroid use were not associated with incident hip fracture risk (Figure 1). Of the lung-disease specific risk factors, post-FEV1% predicted (OR 0.95, 95% CI 0.92–0.99 for each 10% increase), GOLD classification (OR 1.09, 95% CI 1.002–1.19 for each higher stage), ≥ 5% emphysema (OR 1.34, 95% CI 1.06–1.69), mMRC score (OR 1.10, 95% CI 1.03–1.19 for each higher unit score), six-minute walk distance (OR 0.92, 95% CI 0.90–0.95 for each 30 meter increase), BODE index (OR 1.07, 95% CI 1.01–1.13 for each higher unit score), total exacerbations (OR 1.13, 95% CI 1.10–1.16 per exacerbation), and annual exacerbations (OR 1.37, 95% CI 1.21–1.55 per exacerbation) were associated with incident hip fracture (Figure 1). A trend existed between baseline inhaled steroid use and incident hip fracture (OR 1.26, 95% CI 1.00–1.59).
Figure 1:
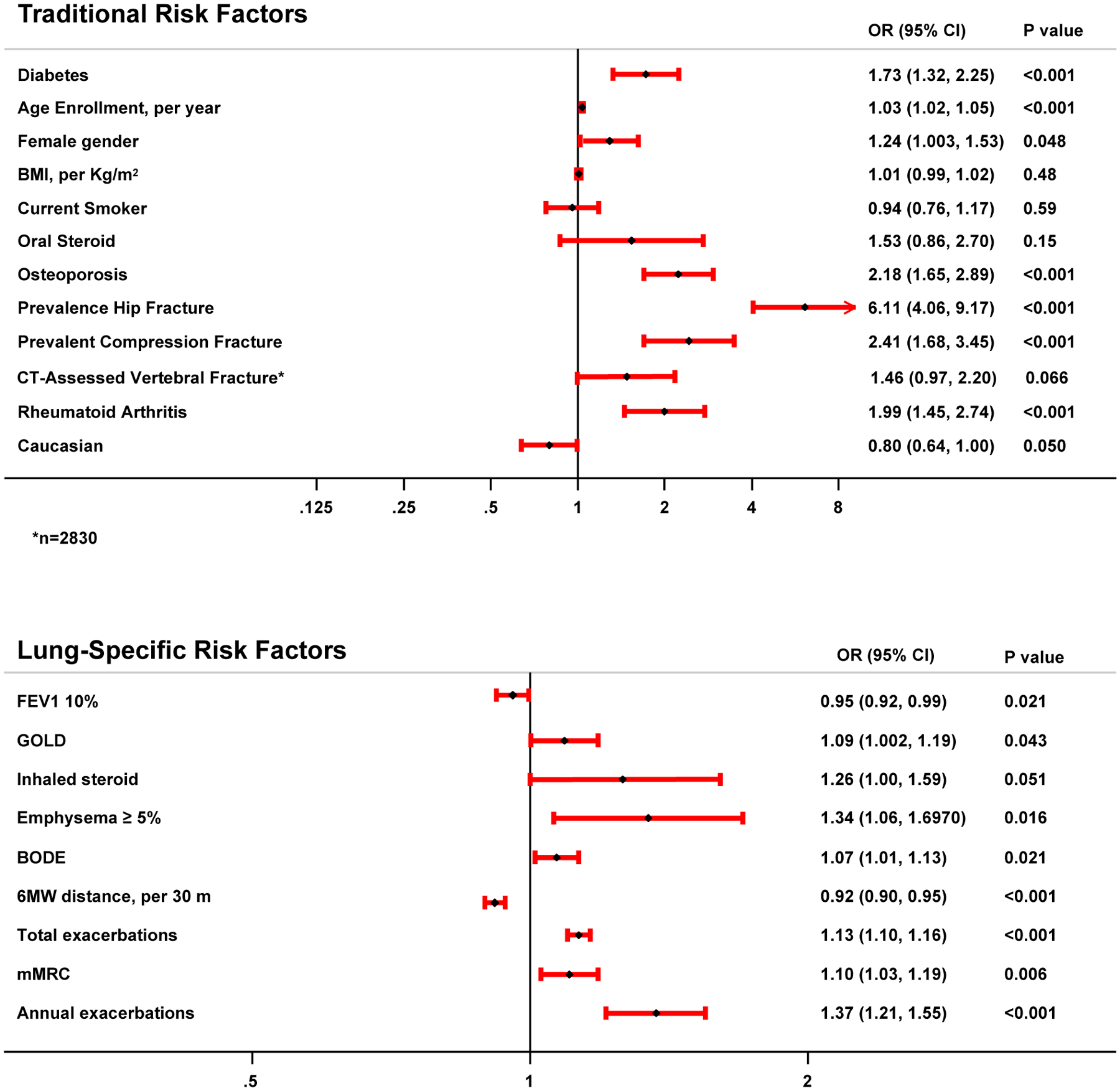
Forest plot depicting traditional and lung-specific risk factors associated with incident hip fracture in univariate logistic regression modeling.
Impact of Lung Disease-Specific Risk Factors on Prediction Models
Predictive modeling for incident hip fracture using traditional osteoporosis risk factors showed similar AUCs for the backward selection, BIC, and BMA models with significant overlap of confidence intervals at 0.66 (0.63–0.69), 0.64 (0.60–0.67) and 0.64 (0.61–0.67) respectively (Table 2). The backward selection model included the greatest number of significant clinical risk factors. Traditional risk factors that were significantly associated with incident hip fracture included older age at enrollment, African American race, reported osteoporosis, prevalent hip and spine fracture, rheumatoid arthritis, and diabetes (Table 2). In sensitivity analyses using CT-assessed vertebral compression fracture in place of self-reported vertebral compression fracture, CT-assessed vertebral compression fracture was not significant in any of the three models. The addition of lung disease-specific risk factors individually to the traditional backward selection model, which was the multivariate model that included the greatest number of significant established risk factors, resulted in similar AUCs. However, the addition of the presence of ≥ 5% quantitative emphysema, six-minute walk distance, and total number of exacerbations individually to the traditional backward selection model improved risk discrimination with IDI values of 0.001 (95% CI 0.0003–0.002), 0.001 (95% CI 0.0001–0.002), and 0.008 (95% CI 0.003–0.013) corresponding to a relative IDI of 12.8%, 6.3%, and 34.6% (Table 3). Likewise, the inclusion of six-minute walk distance and presence of ≥ 5% quantitative emphysema to the model resulted in a significantly greater proportion of individuals being reclassified into a more appropriate risk category with an NRI of 0.043 (SE 0.015) and 0.027 (SE 0.012).
Table 2:
Traditional Population Risk Factors for Hip Fracture, Multivariable Model. Results are Odds ratio (95%CI).
| Backward | BIC | BMA | |
|---|---|---|---|
| Age Enrollment | 1.04 (1.02–1.05) | 1.04 (1.02–1.05) | 1.04 (1.03–1.05) |
| BMI | -- | -- | 1.01 (1.00–1.03) |
| Caucasian | 0.58 (0.45–0.75) | 0.55 (0.43–0.71) | 0.56 (0.43–0.71) |
| Osteoporosis | 1.75 (1.30–2.35) | 1.84 (1.38–2.47) | 1.89 (1.41–2.54) |
| Prevalent Hip Fracture | 4.40 (2.86–6.79) | 5.16 (3.40–7.84) | 5.15 (3.39–7.83) |
| Prevalent Compression Fracture | 1.61 (1.09–2.39) | -- | -- |
| Rheumatoid arthritis | 1.48 (1.06–2.07) | -- | -- |
| Diabetes | 1.54 (1.18–2.03) | -- | -- |
| Smoking | -- | -- | -- |
| Bootstrap AUC (95%CI) | 0.66 (0.63–0.69) | 0.64 (0.60–0.67) | 0.64 (0.61–0.67) |
| P value of H-L test for goodness of fit (higher better) | 0.16 | 0.47 | 0.70 |
Table 3:
Impact of the Addition of Individual Lung-Specific Risk Factors to Prediction Models
| aOR (95%CI)c | Change in BICd | NRI (SE)e | NRI (event) |
NRI (non-event) |
IDI (95% CI)f | Relative IDIg | |
|---|---|---|---|---|---|---|---|
| FEV1, per 10% | 1.00 (0.96–1.004) | 9.2 | 0.005 (0.005) | 0.003 | −0.002 | 0.00001 (−0.0001–0.0001) | 0.5% |
| GOLD | 0.99 (0.90–1.08) | 23.7 | 0.020 (0.014) | −0.054 | −0.074 | −0.0001 (−0.0009–0.0006) | 2.3% |
| Emphysema ≥ 5% | 1.13 (0.88–1.46) | 18.0 | 0.027 (0.012)a | −0.029 | −0.056 | 0.001 (0.0003–0.002)b | 12.8% |
| Inhaled steroids | 0.99 (0.77–1.26) | 14.4 | −0.001 (0.006) | 0.005 | 0.006 | −0.0003 (−0.0029 – −0.0027)b | −3.1% |
| 6-minute walk distance, per 30 meters | 0.999 (0.998–0.9996)b | −9.6 | 0.043 (0.015)b | 0.014 | −0.029 | 0.001 (0.0001–0.002)a | 6.3% |
| Modified Medical Research Council | 1.01 (0.94–1.09) | 0.1 | −0.002 (0.005) | −0.003 | −0.0005 | −0.0001 (−0.001–0.001) | −0.2% |
| Total exacerbations | 1.11 (1.08–1.15)b | −30.8 | 0.026 (0.016) | −0.028 | −0.054 | 0.008 (0.003–0.013)b | 34.6% |
P <0.05
P<0.01
Adjusted Odds ratio (95% CI) for traditional risk factors (age enrollment, race, osteoporosis, prevalent hip or compression fracture, rheumatoid arthritis, diabetes).
Bayesian Information Criteria
Net reclassification index, using a category of 3.0%
Integrated discrimination improvement
Model slope with traditional predictors was 0.0229
Of the three modeling approaches used to determine the best predictive model for incident hip fracture that combines all traditional and lung-specific risk factors into a single model, both the BIC and BMA models had the best discrimination power (AUC = 0.67) and goodness of fit according to the Hosmer-Lemeshow test (p values of 0.70 and 0.49, respectively; Table 4), whereas the backwards selection model demonstrated poor goodness of fit (Hosmer-Lemeshow test p=0.03). Of the prediction variables retained in the BIC and BMA models, only current smoking was not independently associated with incident hip fracture in univariable analysis in our cohort.
Table 4:
Predictive models of Hip Fracture using difference approach for variable selection. Result for each variable is Coefficient (SE).
| BIC | Backward | BMA | |
|---|---|---|---|
| Intercept | −5.054 (0.536) | −5.143 (0.701) | −5.571 (0.485) |
| Age Enrollment, per year | 0.034 (0.008) | 0.041 (0.009) | 0.040 (0.008) |
| Male Gender | -- | -- | -- |
| Caucasian | -- | −0.350 (0.169) | −0.615 (0.158) |
| BMI, per Kg/m2 | -- | -- | -- |
| Current Smoker | 0.323 (0.126) | 0.338 (0.158) | -- |
| Oral steroid | -- | -- | -- |
| Osteoporosis | 0.489 (0.151) | 0.667 (0.180) | 0.646 (0.178) |
| Prevalent Hip Fracture | 1.559 (0.220) | 1.483 (0.278) | 1.563 (0.274) |
| Prevalent Compression Fracture | -- | -- | -- |
| Rheumatoid arthritis | -- | 0.489 (0.206) | -- |
| Diabetes | -- | 0.393 (0.178) | -- |
| FEV1 % | -- | -- | -- |
| GOLD | -- | -- | -- |
| Emphysema ≥ 5% | -- | -- | -- |
| Inhaled steroids | -- | -- | -- |
| BODE | -- | −0.093 (0.044) | -- |
| 6-MW distance (m) | −0.002 (0.0005) | −0.002 (0.001) | -- |
| MMRC | -- | -- | -- |
| Total exacerbations | 0.107 (0.015) | 0.114 (0.020) | 0.113 (0.019) |
| Bootstrap AUC (95%CI) (higher better) | 0.67 (0.63–0.69) | 0.68 (0.65–0.71) | 0.67 (0.63–0.70) |
| P value of H-L test for goodness of fit (higher better) | 0.70 | 0.03 | 0.49 |
DISCUSSION
In this cohort of current and former smokers with detailed lung assessments, nearly 4% of individuals experienced an incident hip fracture over 58,477 participant follow-up years. Incident hip fractures were ascertained by self-report, a strategy that has demonstrated high sensitivity and specificity to detect hip fracture in prior epidemiologic studies.(25) We identified several established and lung-disease specific risk factors independently associated with incident hip fracture in this cohort. Notably, the presence of emphysema on CT scan, six-minute walk distance, and acute exacerbation frequency significantly improved risk discrimination when added individually to models including established fracture risk factors. In contrast to prior studies,(26–28) African American race was associated with increased hip fracture risk. This finding may be attributable to a race-dependent differential impact of lung disease on fracture risk, the increased prevalence of diabetes, where fracture risk is increased despite normal BMD,(29, 30), or treatment disparities in African American participants.
Notably, only 18% and 9% of participants with and without incident hip fracture, respectively, reported a diagnosis of osteoporosis at baseline and even fewer reported the use of anti-resorptive or bone-modifying therapy. We suspect that this is a gross underestimation of osteoporosis prevalence and reflects the known underdiagnosis of osteoporosis in COPD.(10) In fact, of those participants with self-reported hip fracture at baseline, only 26.5% reported a diagnosis of osteoporosis despite the fact that they would all meet the clinical definition of osteoporosis by fracture criteria. Similarly, in the subset of participants with CT-assessed vertebral fractures, nearly one-third had evidence of prevalent fracture, yet only 12.5% reported a diagnosis of osteoporosis. Even in those cohort participants who reported a history of symptomatic vertebral compression fracture at baseline, only 27.1% reported a diagnosis of osteoporosis. These discrepancies between fracture prevalence and self-reported osteoporosis underscore the lack of awareness and failure of physicians to diagnose osteoporosis in this high-risk group of patients. The high prevalence of thoracic CT imaging in the COPD population provides physicians the opportunity to carefully review these studies for fracture and to then proceed with additional osteoporosis screening and management if clinically indicated.
Predictive models that included only traditional risk factors, similar to those used clinically by the FRAX tool, (31) and both traditional and lung-specific risk factors in a single model performed only modestly with AUCs ranging from 0.64 to 0.68. These findings suggest that traditional risk factors may not be optimal for fracture risk prediction in smokers with lung disease. However, studies that have assessed performance of the FRAX tool using “real world” data in some instances have demonstrated similar accuracy. Importantly, we show that CT-determined emphysema, walk distance, and acute exacerbations of COPD improve prediction models when using statistical approaches that are designed to detect small but meaningful improvements in risk discrimination that may not be captured by AUC assessment.(23)
Emerging data suggest that risk factors for osteoporosis in individuals with COPD likely go beyond established risks (i.e. frailty, chronic steroid use), particularly in individuals with milder airflow obstruction in whom low BMD is still prevalent.(3, 6, 14, 15, 32, 33) Studies demonstrate an independent association between CT-determined emphysema and low BMD (13, 14) as well as accelerated bone loss over short-term follow-up intervals.(11) Notably in the COPDGene cohort, we have previously shown that emphysema independently associates with volumetric BMD and prevalent thoracic vertebral fractures.(3) Others have likewise demonstrated an association between acute exacerbation frequency, volumetric thoracic vertebral BMD, and BMD loss over time.(15) This study is the first to show that a history of acute exacerbations is associated with incident hip fractures and that the inclusion of emphysema, six-minute walk distance, and acute exacerbation frequency improves risk discrimination in established models. Current risk assessment models, such as the Fracture Risk Assessment Tool (FRAX),(34) include current smoking and glucocorticoid use but fail to account for the significant lung disease and persistent systemic inflammation that may be present in former smokers, (35, 36) or the cumulative burden from intermittent steroid use in individuals treated for frequent acute exacerbations of COPD, factors likely contributing to incident fracture in our cohort. Of note, while current smoking was retained in our predictive models, this factor was not independently associated with incident hip fracture in our cohort. Although traditional statistical modeling approaches often limit variable inclusion to those associated with the outcome of interest, we chose to include current smoking in our model building as active smoking is widely-accepted and well-established as an osteoporosis risk factor in the literature.(34)
The median age of 63 years and near-equal sex distribution in cohort participants with hip fracture further highlights the inadequacy of guidelines in addressing fracture risk in current and former smokers with lung disease, particularly in men who comprised 46% of those affected. Current screening guidelines recommend BMD assessment in women 65 years of age or older or less than 65 years of age with major risk factors, of which chronic lung disease is not included.(8) However, screening recommendations for men vary. Guidelines that do recommend osteoporosis screening in men focus on screening in men with risk factors, explicitly stating that data are insufficient in men to determine whether respiratory disease increase the risk of low BMD-mediated fracture.(9) Yet, our group and others have shown that lung disease-specific factors such as emphysema and exacerbation frequency are associated with bone loss and prevalent fracture.(3, 11, 14, 15, 32) More permissive osteoporosis screening guidelines, such as those published by the National Osteoporosis Foundation (37) and the American Association of Clinical Endocrinologist/American College of Endocrinology (38), do consider chronic obstructive lung disease to be a significant risk factor for osteoporosis. However, they provide no guidance on which COPD patients are at highest risk for low BMD and warrant earlier screening. Here, we demonstrate that emphysema and exacerbation history as well as six-minute walk distance improve risk discrimination for incident fracture. With the advent of lung cancer screening guidelines recommending annual chest CT imaging in smokers,(39) emphysema status, walk distance, and exacerbation history are easily assessed during a routine office visit. Consideration of these factors may guide early BMD screening in selected high-risk smokers.
A deeper understanding of the mechanisms underlying the associations between walk distance, emphysema, exacerbation frequency and incident fracture is needed to optimize COPD management while avoiding bone loss. Six-minute walk distance provides an overall assessment of functional status and may be influenced by muscle mass, activity level or comorbidities, factors that may impact bone health. Similarities between macrophages and osteoclasts and their respective roles in the pathogenesis of emphysema and osteoporosis implicate them as biologically plausible causal factors in the link between lung and bone. Animal studies have shown that a mutation in the macrophage colony-stimulating factor (M-CSF) gene leads to a defect in both macrophage and osteoclast formation, suggesting a common origin for cells involved in the destruction of lung and bone matrix.(40) Inflammatory mediators, including interleukin (IL) 1, tumor necrosis factor alpha (TNF-α), IL6, and IL17, promote osteoclast-mediated bone resorption and have likewise been implicated in emphysema pathogenesis in both human and animal models.(41–45) Acute exacerbations of COPD are characterized by increased respiratory symptoms and lung inflammation accompanied by heightened systemic inflammation.(46) Although data in COPD are lacking, studies in other chronic lung diseases such as cystic fibrosis demonstrated correlation between circulating inflammatory mediators and elevated markers of bone metabolism during infectious exacerbations that decrease with antimicrobial therapy.(47, 48) The identification of inflammatory pathways activated during periods of both COPD stability and instability and associated with concomitant accelerated bone turnover may lead to precision therapy targeting both disease processes.
This study has several limitations. First, our analysis utilized convenience sampling, leveraging existing clinical data from the COPDGene study. The COPDGene study was designed to determine genetic factors associated with COPD. As such, data regarding important contributors to fracture risk, such as cumulative systemic steroid exposure and falls, were not systematically collected as these were not key factors of interest for the parent cohort study. The mainstay of therapy for acute exacerbations is systemic corticosteroids, duration and dosing of which may vary among individuals. Although oral corticosteroid use at baseline was low and there was no significant difference between groups with respect to reported prior oral or parenteral steroid treatment, information regarding cumulative corticosteroid dosing either prior to enrollment or during longitudinal follow-up was not available. Steroid use during acute exacerbations likely contributed to bone loss and fracture risk. However, studies focusing on the peri-exacerbation period that include detailed assessments of steroid use, systemic inflammatory mediators, physical activity levels, and other factors that may contribute to bone loss are necessary to fully understand how acute exacerbations relate to fracture risk.
The lack of data regarding falls, a key risk factor for fracture, is a limitation of this study. Although we were unable to assess how falls predict incident hip fracture in our cohort, we were able to assess how lung disease-related risk factors associated with osteoporosis in prior studies predict long-term fracture risk, recognizing that both osteoporosis and falls are major predictors of fracture. Osteoporosis is grossly underdiagnosed in current and former smokers with COPD.(10) We would hope that our findings may inform decisions regarding early BMD assessment in select current and former smokers with chronic lung disease, and that primary prevention strategies, including early fall risk assessment, would be implemented when a diagnosis of osteoporosis is established.
Only approximately one-third of the cohort had severe obstructive lung disease at baseline with an annual rate of 0.4 exacerbations per year, limiting the generalizability of our findings to individuals with frequent exacerbations and more severe airflow obstruction. We would anticipate a higher number of incident hip fractures and other established risk factors, such as low BMI, to associate with fracture risk in a more severely diseased smoking population. As this cohort ages and lung disease progresses, we may uncover additional factors associated with fracture risk during longer-term follow-up.
Finally, we focused on incident hip fracture due to the associated morbidity and mortality and availability of this outcome assessment through longitudinal follow-up surveys. Vertebral fractures are likewise associated with significant morbidity, including reductions in lung function and chronic pain. Prevalent vertebral fractures were significant in a subset of this cohort at baseline when assessed objectively on CT scan(3) and associated with lung-disease specific factors including emphysema. However, when we compared self-reported compression fractures to objectively measured vertebral fractures we found the sensitivity of self-report to detect true vertebral fracture quite low at 9%, whereas the specificity was high at 96%. Although associated with incident hip fracture in univariate models despite not being a highly accurate measure, self-reported compression fracture did not impact the final multivariate predictive models. Whether factors associated with incident hip fracture predict incident vertebral fracture also is not known but can be explored as we continue to collect serial chest CT imaging for cohort participants.
Hip fractures contribute to significant morbidity and mortality in smokers with lung disease. We have identified lung-disease specific risk factors that are easily assessed during a routine office visit and improve risk discrimination when added to traditional risk prediction models. A further understanding of the mechanisms underlying these associations is necessary to guide osteoporosis screening strategies and to develop targeted therapy for individuals with COPD-related bone loss.
ACKNOWLEDGEMENTS
Funding
The project described was supported by Award Number U01 HL089897 and Award Number U01 HL089856 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
The COPDGene® project is also supported by the COPD Foundation through contributions made to an Industry Advisory Board comprised of AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Pfizer, Siemens and Sunovion.
DISCLOSURES
JB reports grants from NIH and grants from VA outside the submitted work. SMN and KJS have nothing to disclose. MTD reports grants from NIH during the conduct of the study; grants from Department of Defense, personal fees and other from Boehringer Ingelheim, personal fees and other from GlaxoSmithKline, other from Novartis, personal fees and other from AstraZeneca, other from Yungjin, personal fees and other from PneumRx/BTG, other from Pulmonx, personal fees from Genentech, other from Boston Scientific, personal fees from Quark Pharmaceuticals, personal fees from Mereo, and grants from American Lung Association outside the submitted work. MM reports grants from NIH during the conduct of the study and personal fees from Pfizer outside the submitted work. RPB served on the advisory boards (GlaxoSmithKline, Boehringer Ingelheim, and Mylan Pharmaceuticals) and received research grants from GlaxoSmithKline and Boehringer Ingelheim not related to this manuscript and these activities have not influenced work on this manuscript. EAH is a founder and shareholder in VIDA diagnostics. JDN reports grants from NIH during the conduct of the study; grants from NIH, grants from Siemens Healthineers, and personal fees from VIDA Diagnostics Inc outside the submitted work; a patent Software Algorithm with VIDA issued, a patent Software Algorithm with VIDA pending, and a patent Software Algorithm with University of Iowa issued. APC reports grants from NIH/NHLBI, grants from NIH/NIEHS, grants from NIH/NCATS during the conduct of the study; personal fees from GSK and non-financial support from Vida Diagnostics outside the submitted work. PKS has nothing to disclose. RPB served on the advisory boards (GlaxoSmithKline, Boehringer Ingelheim, and Mylan Pharmaceuticals) and received research grants from GlaxoSmithKline and Boehringer Ingelheim not related to this manuscript and these activities have not influenced work on this manuscript. EAR has nothing to disclose.
Footnotes
Publisher's Disclaimer: This is the accepted version of the following article: Bon J, Nouraie SM, Smith KJ, Dransfield MT, McDonald ML, Hoffman EA, Newell JD Jr, Comellas AP, Saha PK, Bowler RP, Regan EA. Lung-Specific Risk Factors Associated With Incident Hip Fracture in Current and Former Smokers. J Bone Miner Res 2020;35(10)1952-61., which has been published in final form at: https://asbmr.onlinelibrary.wiley.com/doi/10.1002/jbmr.4103
No supplemental data has been included with this submission.
Contributor Information
Jessica Bon, University of Pittsburgh, Department of Medicine, Division of Pulmonary, Allergy and Critical Care Medicine and VA Pittsburgh Healthcare System, Pittsburgh, PA;.
Seyed Mehdi Nouraie, University of Pittsburgh, Department of Medicine, Division of Pulmonary, Allergy and Critical Care Medicine, Pittsburgh, PA;.
Kenneth J. Smith, University of Pittsburgh, Department of Medicine, Division of General Internal Medicine, Pittsburgh, PA;.
Mark T Dransfield, University of Alabama at Birmingham, Lung Health Center, Department of Medicine, Division of Pulmonary, Allergy and Critical Care Medicine;.
Merry-Lynn McDonald, University of Alabama at Birmingham, Lung Health Center, Department of Medicine, Division of Pulmonary, Allergy and Critical Care Medicine;.
Eric A. Hoffman, University of Iowa, Departments of Radiology, Internal Medicine and Biomedical Engineering, Iowa City, IA;.
John D. Newell, Jr., University of Iowa, Departments of Radiology, Internal Medicine and Biomedical Engineering, Iowa City, IA;.
Alejandro P. Comellas, University of Iowa, Department of Internal Medicine, Division of Pulmonary, Critical Care and Occupational Medicine, Iowa City, IA;.
Punam K. Saha, University of Iowa, Departments of Radiology, Internal Medicine and Biomedical Engineering, Iowa City, IA;.
Russell P. Bowler, National Jewish Health, Department of Medicine, Division of Pulmonary, Critical Care and Sleep Medicine, Denver, CO;.
Elizabeth A. Regan, National Jewish Health, Department of Medicine, Division of Rheumatology, Denver, CO;.
REFERENCES
- 1.Gershon AS, Thiruchelvam D, Chapman KR, Aaron SD, Stanbrook MB, Bourbeau J, et al. Health Services Burden of Undiagnosed and Overdiagnosed COPD. Chest. 2018;153(6):1336–46. [DOI] [PubMed] [Google Scholar]
- 2.Divo M, Cote C, Torres JPd, Casanova C, Marin JM, Pinto-Plata V, et al. Comorbidities and Risk of Mortality in Patients with Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2012;186(2):155–61. [DOI] [PubMed] [Google Scholar]
- 3.Jaramillo JD, Wilson C, Stinson DJ, Lynch DA, Bowler RP, Lutz S, et al. Reduced Bone Density and Vertebral Fractures in Smokers. Men and COPD Patients at Increased Risk. Ann Am Thorac Soc. 2015;12(5):648–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sin DD, Man JP, Man SF. The risk of osteoporosis in Caucasian men and women with obstructive airways disease. Am J Med. 2003;114(1):10–4. [DOI] [PubMed] [Google Scholar]
- 5.Mesbahul Islam SAHM, Bari MZJ, Islam AFMN. Comparison of osteoporosis in male smokers with or without COPD. Eur Respir J. 2016;48. [Google Scholar]
- 6.Bon J, Fuhrman CR, Weissfeld JL, Duncan SR, Branch RA, Chang C-CH, et al. Radiographic emphysema predicts low bone mineral density in a tobacco-exposed cohort. Am J Respir Crit Care Med. 2011;183(7):885–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Vries F, van Staa TP, Bracke MSGM, Cooper C, Leufkens HGM, Lammers JWJ. Severity of obstructive airway disease and risk of osteoporotic fracture. Eur Respir J. 2005;25(5):879–84. [DOI] [PubMed] [Google Scholar]
- 8.Force UPST. Screening for Osteoporosis to Prevent Fractures: US Preventive Services Task Force Recommendation Statement USPSTF Recommendation Statement: Screening for Osteoporosis to Prevent FracturesUSPSTF Recommendation Statement: Screening for Osteoporosis to Prevent Fractures. JAMA. 2018;319(24):2521–31. [DOI] [PubMed] [Google Scholar]
- 9.Qaseem A, Snow V, Shekelle P, Hopkins R Jr., Forciea MA, Owens DK. Screening for osteoporosis in men: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;148(9):680–4. [DOI] [PubMed] [Google Scholar]
- 10.Regan EA, Radcliff TA, Henderson WG, Cowper Ripley DC, Maciejewski ML, Vogel WB, et al. Improving hip fractures outcomes for COPD patients. COPD. 2013;10(1):11–9. [DOI] [PubMed] [Google Scholar]
- 11.Bon J, Zhang Y, Leader JK, Fuhrman C, Perera S, Chandra D, et al. Radiographic Emphysema, Circulating Bone Biomarkers, and Progressive Bone Mineral Density Loss in Smokers. Ann Am Thorac Soc. 2018;15(5):615–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petraglia A, Leader JK, Gingo M, Fitzpatrick M, Ries J, Kessinger C, et al. Emphysema is associated with thoracic vertebral bone attenuation on chest CT scan in HIV-infected individuals. Plos One. 2017;12(4):e0176719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohara T, Hirai T, Muro S, Haruna A, Terada K, Kinose D, et al. Relationship between pulmonary emphysema and osteoporosis assessed by CT in patients with COPD. Chest. 2008;134(6):1244–9. [DOI] [PubMed] [Google Scholar]
- 14.Kiyokawa H, Muro S, Oguma T, Sato S, Tanabe N, Takahashi T, et al. Impact of COPD exacerbations on osteoporosis assessed by chest CT scan. COPD. 2012;9(3):235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Høidrup S, Prescott E, Sørensen TI, Gottschau A, Lauritzen JB, Schroll M, et al. Tobacco smoking and risk of hip fracture in men and women. Int J Epidemiol. 2000;29(2):253–9. [DOI] [PubMed] [Google Scholar]
- 16.Kanis JA, Johnell O, Oden A, Johansson H, De Laet C, Eisman JA, et al. Smoking and fracture risk: a meta-analysis. Osteoporos Int. 2005;16(2):155–62. [DOI] [PubMed] [Google Scholar]
- 17.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7(1):32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38. [DOI] [PubMed] [Google Scholar]
- 19.Bhatt SP, Washko GR, Hoffman EA, Newell JD Jr., Bodduluri S, Diaz AA, et al. Imaging Advances in Chronic Obstructive Pulmonary Disease. Insights from the Genetic Epidemiology of Chronic Obstructive Pulmonary Disease (COPDGene) Study. Am J Respir Crit Care Med. 2019;199(3):286–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffman EA, Ahmed FS, Baumhauer H, Budoff M, Carr JJ, Kronmal R, et al. Variation in the percent of emphysema-like lung in a healthy, nonsmoking multiethnic sample. The MESA lung study. Ann Am Thorac Soc. 2014;11(6):898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoeting JA, Madigan D, Raftery AE, Volinsky CT. Bayesian model averaging: a tutorial (with comments by M. Clyde, David Draper and E. I. George, and a rejoinder by the authors. Statist Sci. 1999;14(4):382–417. [Google Scholar]
- 22.Pencina MJ, D’Agostino RB Sr., D’Agostino RB Jr., Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Statistics in medicine. 2008;27(2):157–72; discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 23.Pepe MS. Problems with risk reclassification methods for evaluating prediction models. Am J Epidemiol. 2011;173(11):1327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ivers RQ, Cumming RG, Mitchell P, Peduto AJ. The accuracy of self-reported fractures in older people. J Clin Epidemiol. 2002;55(5):452–7. [DOI] [PubMed] [Google Scholar]
- 25.Looker AC, Borrud LG, Dawson-Hughes B, Shepherd JA, Wright NC. Osteoporosis or low bone mass at the femur neck or lumbar spine in older adults: United States, 2005–2008. NCHS data brief. 2012(93):1–8. [PubMed] [Google Scholar]
- 26.Looker AC, Melton LJ 3rd, Borrud LG, Shepherd JA. Lumbar spine bone mineral density in US adults: demographic patterns and relationship with femur neck skeletal status. Osteoporos Int. 2012;23(4):1351–60. [DOI] [PubMed] [Google Scholar]
- 27.Bohannon AD, Hanlon JT, Landerman R, Gold DT. Association of race and other potential risk factors with nonvertebral fractures in community-dwelling elderly women. Am J Epidemiol. 1999;149(11):1002–9. [DOI] [PubMed] [Google Scholar]
- 28.Looker AC, Eberhardt MS, Saydah SH. Diabetes and fracture risk in older U.S. adults. Bone. 2016;82:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strotmeyer ES, Cauley JA, Schwartz AV, Nevitt MC, Resnick HE, Bauer DC, et al. Nontraumatic fracture risk with diabetes mellitus and impaired fasting glucose in older white and black adults: the health, aging, and body composition study. Arch Int Med. 2005;165(14):1612–7. [DOI] [PubMed] [Google Scholar]
- 30.Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19(4):385–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldshtein I, Ish-Shalom S, Leshno M. Impact of FRAX-based osteoporosis intervention using real world data. Bone. 2017;103:318–24. [DOI] [PubMed] [Google Scholar]
- 32.Suh YJ, McDonald MN, Washko GR, Carolan BJ, Bowler RP, Lynch DA, et al. Lung, Fat and Bone: Increased Adiponectin Associates with the Combination of Smoking-Related Lung Disease and Osteoporosis. Chronic Obstr Pulm Dis. 2018;5(2):134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogura-Tomomatsu H, Asano K, Tomomatsu K, Miyata J, Ohmori N, Kodama M, et al. Predictors of osteoporosis and vertebral fractures in patients presenting with moderate-to-severe chronic obstructive lung disease. COPD. 2012;9(4):332–7. [DOI] [PubMed] [Google Scholar]
- 34.McCloskey EV, Fau Johansson H - Oden A, Fau Oden A - Kanis JA, Kanis JA. From relative risk to absolute fracture risk calculation: the FRAX algorithm. (1544–2241 (Electronic)) [DOI] [PubMed] [Google Scholar]
- 35.Gan WQ, Man SF, Sin DD. The interactions between cigarette smoking and reduced lung function on systemic inflammation. Chest. 2005;127(2):558–64. [DOI] [PubMed] [Google Scholar]
- 36.Frohlich M, Sund M, Lowel H, Imhof A, Hoffmeister A, Koenig W. Independent association of various smoking characteristics with markers of systemic inflammation in men. Results from a representative sample of the general population (MONICA Augsburg Survey 1994/95). Eur Heart J. 2003;24(14):1365–72. [DOI] [PubMed] [Google Scholar]
- 37.Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int. 2014;25(10):2359–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Camacho PM, Petak SM, Binkley N, Clarke BL, Harris ST, Hurley DL, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis - 2016. Endocrine Practice. 2016;22(Supplement 4):1–42. [DOI] [PubMed] [Google Scholar]
- 39.The National Lung Screening Trial Research T. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. New Engl J Med. 2011;365(5):395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Churg A, Zhou S, Wang X, Wang R, Wright JL. The Role of Interleukin-1{beta} in Murine Cigarette Smoke-Induced Emphysema and Small Airway Remodeling. Am J Respir Cell Mol Biol. 2009;40(4):482–90. [DOI] [PubMed] [Google Scholar]
- 41.Xiong Z, Leme AS, Ray P, Shapiro SD, Lee JS. CX3CR1+ Lung Mononuclear Phagocytes Spatially Confined to the Interstitium Produce TNF-Œ± and IL-6 and Promote Cigarette Smoke-Induced Emphysema. J Immunol. 186(5):3206–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Churg A, Dai J, Tai H, Xie C, Wright JL. Tumor Necrosis Factor-{alpha} Is Central to Acute Cigarette Smoke-induced Inflammation and Connective Tissue Breakdown. Am J Respir Crit Care Med. 2002;166(6):849–54. [DOI] [PubMed] [Google Scholar]
- 43.Ruwanpura SM, McLeod L, Miller A, Jones J, Bozinovski S, Vlahos R, et al. Interleukin-6 Promotes Pulmonary Emphysema Associated with Apoptosis in Mice. Am J Respir Cell Mol Biol. 45(4):720–30. [DOI] [PubMed] [Google Scholar]
- 44.Chen K, Pociask DA, McAleer JP, Chan YR, Alcorn JF, Kreindler JL, et al. IL-17RA is required for CCL2 expression, macrophage recruitment, and emphysema in response to cigarette smoke. PloS One. 2011;6(5):e20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pinto-Plata VM, Livnat G, Girish M, Cabral H, Masdin P, Linacre P, et al. Systemic Cytokines, Clinical and Physiological Changes in Patients Hospitalized for Exacerbation of COPD. Chest. 2007;131(1):37–43. [DOI] [PubMed] [Google Scholar]
- 46.Aris RM, Stephens AR, Ontjes DA, Denene Blackwood A, Lark RK, Hensler MB, et al. Adverse alterations in bone metabolism are associated with lung infection in adults with cystic fibrosis. Am J Respir Crit Care Med. 2000;162(5):1674–8. [DOI] [PubMed] [Google Scholar]
- 47.Shead EF, Haworth CS, Gunn E, Bilton D, Scott MA, Compston JE. Osteoclastogenesis during Infective Exacerbations in Patients with Cystic Fibrosis. Am J Respir Crit Care Med. 2006;174(3):306–11. [DOI] [PubMed] [Google Scholar]
- 48.Lowe KE, Mansfield KE, Delmestri A, Smeeth L, Roberts A, Abuabara K, et al. Atopic eczema and fracture risk in adults: A population-based cohort study. J All Clin Immunol. 2019. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamen DL, Alele JD. Skeletal manifestations of systemic autoimmune diseases. Curr Opin Endocrinol Diabetes Obes. 2010;17(6):540–5. [DOI] [PubMed] [Google Scholar]


