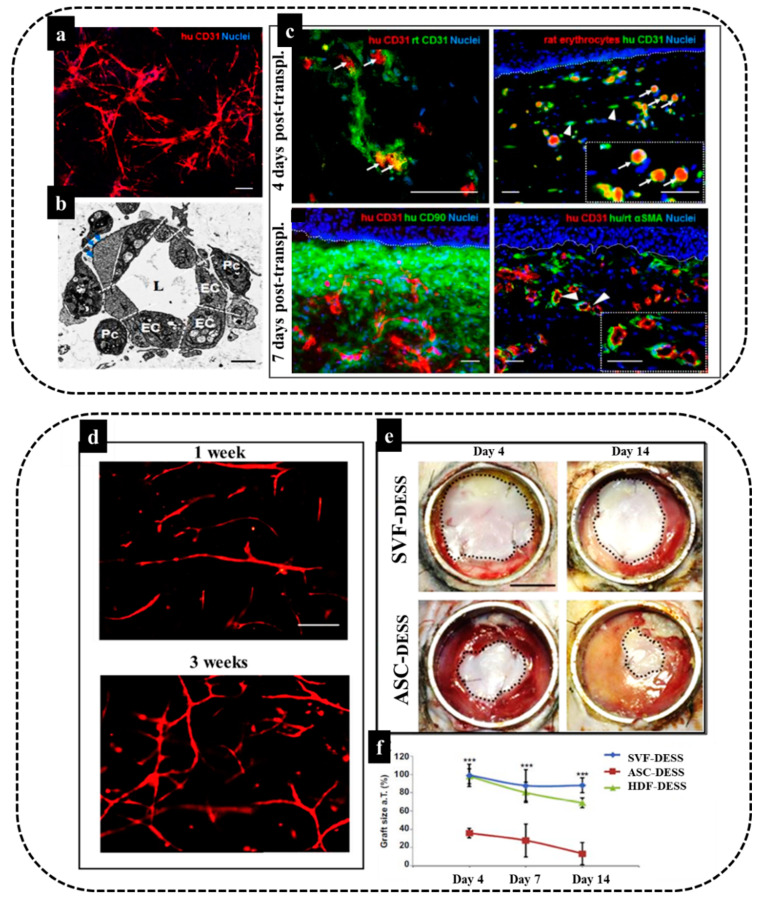
Figure 5.
Engineering of dermo-epidermal skin substitutes with adipose-SVF cells. (a,b) Endothelial cells derived from freshly isolated SVF and perivascular cells in fibrin–collagen type I hydrogel demonstrate in vitro tubular-like structure formation and in vivo anastomosis with the host vasculature. Formation of a complex network of interconnected capillaries after 21 days in culture. Human bioengineered capillaries are stained for human-specific CD31 marker (red) and cell nuclei with Hoechst (blue) and transmission electron microscopy showing a cross-section of an in vitro grown capillary. Note the presence of a central lumen (L), which is surrounded by multiple ECs (EC) covered by pericytes (Pc). The deposition of basement membrane (BM) (blue arrows) was also detected. (c) Establishment of a functional connection (white arrows) between human CD31-positive capillaries (red) and rat CD31-positive capillaries (green) already 4 days post-transplantation. This connection was further confirmed by the presence of rat erythrocytes (red autofluorescence) in the lumina of human CD31-positive capillaries (green) (white arrows). The inset shows a magnification of the area indicated by white arrows. White arrowheads indicate nonperfused human capillaries. Moreover, representative section of a highly vascularized human dermo-epidermal skin substitute after 7 days post-transplantation is demonstrated. The engineered capillaries are visualized by the human specific CD31 antibody costained by human CD90 marker delineating the human dermal compartment. Staining for human/rat aSMA (pericyte marker) reveals that the majority of transplanted capillaries were already covered by pericytes in vivo. Hoechst stains the nuclei blue. White dotted lines indicate the dermo-epidermal junction [8]. (d) Optimization of vascular network formation in vitro to determine the optimal culture time for maximal in vitro capillary network formation, fibrin hydrogels containing SVF stained using a human specific CD31 antibody at one and three weeks of culture, (e) The SVF–DESS capillary plexus reduces shrinkage and accelerates the establishment of tissue homeostasis. Black dotted lines indicate the area of each skin transplant used for planimetry analysis and (f) the skin graft coverage area in was significantly improved in SVF–DESS as compared to control groups (*** p < 0.001) [4].