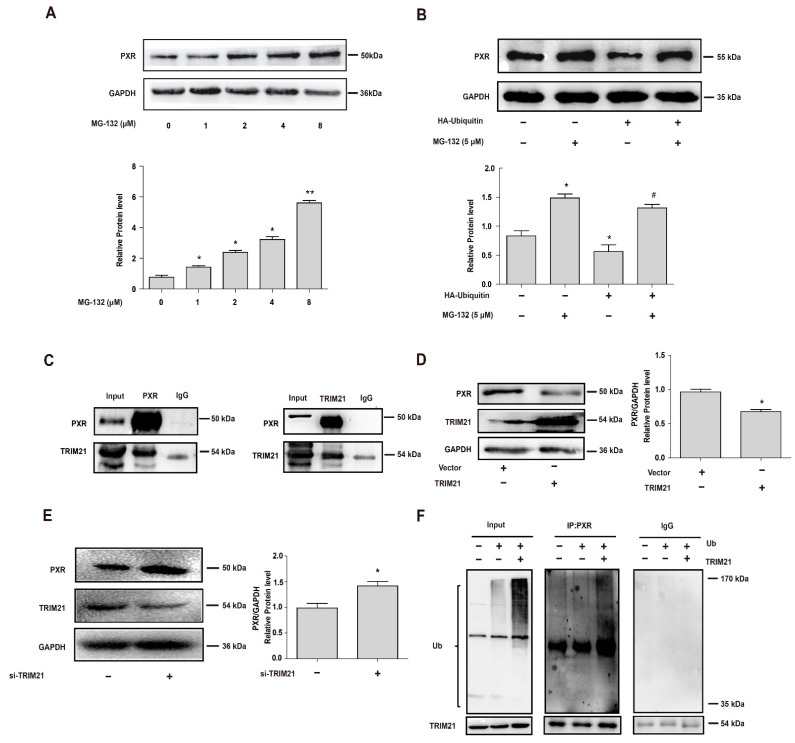
Figure 1.
The E3 ligase TRIM21 binds to PXR and mediates its ubiquitination and degradation. (A) HepG2 cells were treated with MG-132 (0, 1, 2, 4 or 8 μM) for 6 h in the presence of CHX (10 μM), and PXR protein levels were determined by Western blot. (B) HEK 293T cells were transfected with HA-ubiquitin for 24 h and then treated with or without MG-132 5 μM for 24 h, and PXR protein levels were determined by Western blot. (C) Interaction of TRIM21 with PXR. HEK 293T cells were transfected with PXR and TRIM21 constructs as indicated. Cell extracts were immunoprecipitated using PXR antibody or IgG and probed with TRIM21 (left). Reciprocally, the extracts were immunoprecipitated with TRIM21 antibody or IgG and probed with PXR antibody (right). (D) Ectopic overexpression of TRIM21 in HepG2 cells decreased endogenous PXR levels. HepG2 cells were transfected with TRIM21 construct, and the protein level of PXR was analyzed by Western blot 48 h later. (E) HepG2 cells were transfected with siTRIM21 and siRNA-control for 48 h. Cell lysates were prepared and subjected to Western blot to determine the expression of PXR and TRIM21, respectively. (F) TRIM21 ubiquitinates PXR. HEK 293T cells were transfected with corresponding vector or HA-Ubiquitin with or without the co-transfection of TRIM21 as indicated. After 24 h, cells were treated with MG-132 (5 μM) for 24 h. Cell lysates were immunoprecipitated with PXR antibody (IP: PXR) or IgG, subjected to Western blot, and probed with anti-HA antibody to detect ubiquitination of PXR. The ubiquitinated ladder was more pronounced when the cells were transfected with ubiquitin and TRIM21 together, compared with those transfected with ubiquitin alone. Experiments described in this figure were repeated independently at least three times, and data are expressed as mean ± SEM (n = 3). * p < 0.05, ** p < 0.01 versus control; # p < 0.05 versus HA-ubiquitin overexpression group.