Abstract
A number of gut epithelial cells derived immunological factors such as cytokines and chemokines, which are stimulated by the gut microbiota, can regulate host immune responses to maintain a well-balance between gut microbes and host immune system. Multiple specialized immune cell populations, such as macrophages, dendritic cells (DCs), innate lymphoid cells, and T regulatory (Treg) cells, can communicate with intestinal epithelial cells (IEC) and/or the gut microbiota bi-directionally. The gut microbiota contributes to the differentiation and function of resident macrophages. Situated at the interface between the gut commensals and macrophages, the gut epithelium is crucial for gut homeostasis in microbial recognition, signaling transformation, and immune interactions, apart from being a physical barrier. Thus, three distinct but interactive components—macrophages, microbiota, and IEC—can form a network for the delicate and dynamic regulation of intestinal homeostasis. In this review, we will discuss the crucial features of gut microbiota, macrophages, and IEC. We will also summarize recent advances in understanding the cooperative and dynamic interactions among the gut microbiota, gut macrophages, and IEC, which constitute a special network for gut homeostasis.
Keywords: gut macrophage, differentiation, tolerance, epithelial barrier, bacteria recognition, immunomodulation, proliferation
1. Introduction
The gut microbiota contains bacteria, fungi, archaea, and protozoa. They play a critical role in human health and diseases [1]. The most abundant phyla in the human intestine are Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria [2]. Commensal bacteria promote intestinal immune system maturity [3] and protect against pathogen colonization [4]. Lactic acid bacteria (Firmicutes phylum) are marked as probiotic bacteria that are beneficial for human health. Despite the commensal benefits of the gut microbiota, the presence of aberrant microbes or perturbation of the gut microbiota can lead to intestinal disorder and inflammation [5]. Escherichia coli (E. coli) can cause infection or diseases under certain conditions. These aberrant microorganisms may be present within a healthy microbiota. The microbiota commensal microbes can control the expansion of these microorganisms. These gut microbiota can be altered by host genetics, overuse of antibiotics, and changes in diet [6].
Tissue resident macrophages are highly heterogeneous. Each subset of macrophages possesses a unique transcriptome to fulfill niche-specific functions [7,8]. Gut macrophages are strategically underlying the intestinal epithelium, mostly in the lamina propria (LP) [9,10]. The macrophages in LP contribute to host defense and barrier integrity, as well as the constitutive secretion of interleukin (IL)-10 for the maintenance of FoxP3+ T regulatory cells (Treg) [11]. The gut macrophages can be continually replenished by bone marrow derived monocytes in a chemokine receptor 2 (CCR2) dependent manner [12]. These gut macrophages are also derived from embryonic progenitors in the yolk sac and/or fetal liver [13].
The gut epithelium is composed of a single layer of cells, thereby forming crypt villus units [14]. This single layer epithelium alongside mucus segregates the core body from the luminal contents, providing a physical barrier. Furthermore, intestinal epithelial cells (IEC) secrete antimicrobial peptides (AMPs) and cytokines to coordinate the regulation of the active immune responses to infection, inflammation, and homeostasis [15]. In general, the epithelium consists of stem cells, absorptive enterocytes, Paneth cells, goblet cells, and enterochromaffin cells [16]. However, the mucus content and composition correlate with the composition of the gut microbiota in the small and large bowel, which is markedly different in both gut sections.
In the intestinal mucosal environment, gut epithelial cell-derived factors, including cytokines such as IL-18 and chemokines, which are stimulated by the gut microbiota, modulate host immune responses to maintain a good balance between the gut microbes and the host immune system. However, multiple specialized immune cell populations such as macrophages, innate lymphoid cells, dendritic cells (DCs), and Treg cells can communicate with the IEC and/or the gut microbiota bidirectionally [17,18]. The luminal content, such as commensal microbes, can also be sampled by mucosal macrophages. Thus, three distinct but interactive components—gut microbiota, immune cells such as macrophages, and IEC—can form a special network for the delicate and dynamic regulation of intestinal homeostasis (Figure 1). In this review, we will discuss crucial features of the gut microbiota, macrophages, and IEC. We will also summarize recent advances in understanding the cooperative and dynamic interactions of the network comprised of the gut microbiota, macrophages, and IEC.
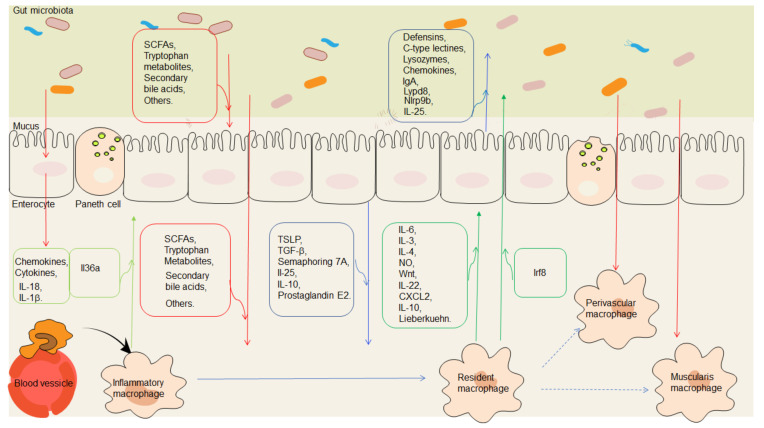
Figure 1.
A special network comprised of macrophages, the gut microbiota, and epithelial cells for gut homeostasis. Immunological mediators, including cytokines and chemokines secreted from the gut epithelial cells stimulated by gut microbiota, such as IL-18 and IL-1β, modulate host immune responses and maintain a well-balanced relationship between gut microbes and the host immune system. The metabolites of the gut microbiota, such as short-chain fatty acids (SCFAs), tryptophan metabolites, secondary bile acids, and polyamines, regulate the proliferation and function of the gut epithelial cells. The metabolites of the gut microbiota, such as SCFAs, tryptophan metabolites, secondary bile acids, UroA/UAS03, polysaccharide, and polyamine can promote the differentiation of macrophages into resident macrophages. Substances such as defensins, c-type lectins, lysozymes, chemokines, Ly6/Plaur domain-containing 8 (Lypd8), Nlrp9b, and interleukin (IL)-25, produced by the gut epithelial cells, especially Paneth cells, also have effects on the gut microbiota. Intestinal epithelial cells (IEC) also produce factors such as thymic stromal lymphopoietin (TSLP), TGFβ, semaphoring 7A, transforming growth factor (TGF-β), retinoic acid, IL-25, and apoptotic cells to promote the macrophages into resident macrophages. Conversely, macrophages can generate some factors such as IL-6, IL-3, IL-4, nitric oxide (NO), Wnt, IL-10, and Lieberkuehn to regulate the proliferation and function of the gut epithelial cells. Meanwhile, macrophages can directly or indirectly produce effects on the gut microbiota. Recent studies have also found effects of the gut microbiota on the perivascular macrophages and muscularis macrophages. Red lines with arrows indicate that the effects of the gut microbiota or their metabolites in the gut contents on the macrophages or gut epithelial cells. Blue lines with arrows indicate that effects of the gut epithelial cell derived factors on the macrophages or gut microbiota. Green lines with arrows indicate the effects of the macrophage derived factors on the gut epithelial cells or gut microbiota. Boxes indicate the components from the gut microbiota, gut epithelial cells, or macrophages.
2. Macrophages and Gut Microbiota
Intestinal macrophages reside either within the LP or the muscle layer. Muller et al. [9] discussed recent advances regarding the origin, phenotype, and function of the macrophages residing in the different layers of the intestine during homeostasis. LP macrophages (LPMs) can be further subdivided into mucosal and submucosal LPMs [19]. Mucosal LPMs can line the intestinal epithelium and vasculature in the murine small and large intestines [20,21]. They contribute to the host defense and barrier integrity, as well as interleukin (IL)-10, which promotes the maintenance of FoxP3+ Treg [22]. Perivascular macrophages in the small intestine and colon mainly participate in the regulation of the vasculature [19,21]. Submucosal LPMs can sense luminal antigens to protect the mucosa from enteropathogens, whereas macrophages residing in the muscularis are essential for tissue homeostasis. The interaction between muscularis macrophage and neurons controls intestinal motility and protects gut tissues during injury and infection. However, it also creates tissue damage in gastrointestinal disorders, such as gastroparesis [23]. These subsets of macrophages with different transcriptional profiles are further confirmed through single-cell RNA sequencing technologies [24,25,26,27] (Figure 2). There is a “monocyte waterfall” from circulation to the intestine in order to maintain the macrophage pool in the gut in a CCR2 dependent manner in the murine colon [12]. These monocytes are identified as the ly6chi CX3CR1int MHCII− subset and exhibit pro-inflammatory properties as they act in the circulation. In the resting intestine, they terminally become mature resident ly6clow/− CX3CR1hi MHCIIhi macrophages, which express IL-10 and maintain intestinal homeostasis. These macrophages are the main cell source for the tolerogenic IL-10. In addition, two intrinsic sources also exist, embryo derived F4/80hi CD11blow macrophages and yolk sac derived macrophages, in the colon or small intestine [12,13]. Fate mapping and single cell RNA sequences have identified these macrophages that arise from both embryonic precursors, which express different transcriptional profiles in the murine small intestine [19].
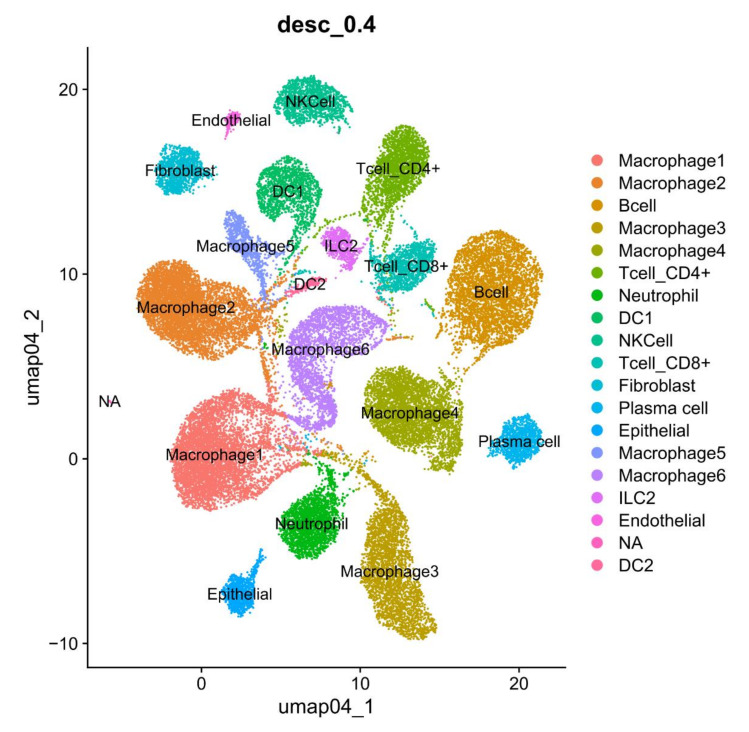
Figure 2.
Subpopulations of macrophages in the colon tissues by scRNA-seq analyses. CD45+CD11b+ cells in the colon tissues were sorted and then used as scRNA-seq analyses.
2.1. Gut Microbiota and Resident Macrophages
2.1.1. Effects of Gut Microbiota on the Resident Macrophages
Gut microbes are essential for the differentiation and function of resident macrophages. In germ-free (GF) mice, the number, phenotype, and function of the gut macrophages are impaired; but they can be restored by microbial colonization from adult mice [28]. IL-1β, IL-12, and IL-10 expressions in small intestinal macrophages are reduced in microbial depleted conditions [29,30,31]. The commensal microbiota can promote the development of two major subsets, CD11c+CD121b+ and CD11c− CD206hi, resident macrophages in the colon [25]. The altered intestinal microbiota can potentially cause a shift between inflammatory macrophages to tolerant macrophages in the colon or small intestine [32,33,34]. High-fat diet-induced dysbiosis mediates macrophage chemotactic protein (MCP)-1/CCR2 axis-dependent M2 macrophage polarization in the murine small intestine [34]. Notably, several bacteria strains, which can induce differentiation and function of resident macrophages in the colon, have been identified, such as Enterococcus faecalis (E. faecalis), Streptococcus gallolyticus (S. gallolyticus), and E. coli [35,36]. Fusobacterium nucleatum (F. nucleatum) also triggers macrophage activation through TLR4 and subsequent IL-6/STAT3/c-MYC signaling, supporting its anti-inflammatory polarization in the colon [37].
2.1.2. Effects of Gut Microbiota Metabolites on Resident Macrophages
(1) Short-chain fatty acids (SCFAs), including acetate, propionate, and butyrate, are the major metabolic products of the gut microbiota digestion of non-absorbable dietary fiber and resistant starches [38]. Acetate is produced from pyruvate in two different ways, acetyl-CoA by enteric bacteria and Wood−Ljungdahl by acetogens, whereas propionate can be produced by the lactate pathway by Firmicutes and the succinate pathway by Bacteroidetes [39]. Eubacterium, Clostridia, Ruminococcaceae, and Firmicutes are the main producers of butyrate from Acetyl-CoA [39]. These SCFAs play an important role in inducing resident macrophages in the colon [40,41,42]. Treatment with the butyrate directly causes metabolic reprogramming of the intestinal macrophages and prevents macrophage from dysfunction [42]. SCFAs exert anti-inflammatory effects through binding to G-protein-coupled receptor 43 (GPR43) [43] and GPR109a expressed in the macrophages [44]. The butyrate can act as a histone deacetylase (HDAC) inhibitor [45] and directly suppress NF-κB activation to regulate the intestinal macrophage function in the colon [46], exerting its anti-inflammatory effects. In addition, butyrate also inhibits HDAC3 to reduce the activation of the mammalian target of rapamycin (mTOR) and glycolysis, which contribute to the antimicrobial function of macrophages [47].
(2) Tryptophan (TRP) metabolites can be metabolized into indole, indole-3-acrylic acid (IA), indole-3-acetic acid (IAA), indole-3-carboxalaldehyde (ICAld), indole-3-propionic acid (IPA), indole-3-lactic acid (ILA), indole-3-acetonitrile (IACN), indole-3-ethanol (IE), and indole-3-carboxylic acid (ICA) [48,49]. Some bacterial species such as Proteus vulgaris can convert TRP into indole by using tryptophanase [50]. Clostridium sporogenes and Peptostreptococcus spp. produce IA [51,52]. Clostridium sporogenes and Rominococcus gnavus produce tryptamine [53]. Recent evidence has shown that inflammation is related with the alterations in the metabolism of TRP. Kynurenine (Kyn)/Trp is also used as an indicator of the progression of inflammation [54]. Notably, both metabolites tryptamine and indole-3-acetate (I3A) can reduce the fatty-acid- and LPS-stimulated production of pro-inflammatory cytokines in the macrophages in the colon [55].
(3) Secondary bile acids. Primary bile acids are synthesized by hepatocytes. Approximately 5% of bile acids are converted into secondary bile acids by microbiota species in the cecum and colon. Lithocholic acid (LCA) and deoxycholate (DCA) are the two major types generated through 7a-dehydroxylation of cholic acid (CA) and chenodeoxycholic acid (CDCA), respectively. Bacteroides, Bifidobacteria, and Lactobacillus can synthesize secondary bile acids from primary bile acids [56,57]. Interestingly, the bile acid-activated receptors (FXR, vitamin D receptor (VDR), liver X receptor (LXR), GPBAR1, and pregnane X receptor (PXR)) can be detected in myeloid cells [58]. Furthermore, the binding of the bioactive molecules to receptors such as the G protein-coupled bile acid receptor 1 (GPBAR1 or Takeda G-protein receptor 5(TGR5)) and farnesoid-X-Receptor (FXR) on the macrophages may exert an anti-inflammatory role [59,60]. TGR5 also regulates the M1/M2 phenotype of the intestinal macrophages [61]. Bile acid inhibits LPS mediated proinflammatory cytokines in a TGR5-dependent manner in the macrophages [62]. The knockout of TGR5 in mice can accelerate LPS-mediated inflammation in the liver and abolish the suppressive effect of the TGR5 agonist on inflammatory cytokines [63]. A TGR5-specific semisynthetic bile acid suppresses the foam cell formation of macrophages by decreasing the uptake of low dense lipoprotein (LDL) [64].
(4) Other metabolites. Branched-chain amino acids (BCAAs) can inhibit LPS-induced nitric oxide (NO) and IL-6, and decrease the damage by H2O2 in macrophages in vitro [65]. Symbiotic polyamine can cause increased anti-inflammatory macrophages in the colon [66]. Quercetin-3-glucuronide inhibits the expression of scavenger receptor class A type 1 (SR-A1) and CD36, and also suppresses the formation of foam cells in RAW 264.7 cells [67]. Recent studies have reported that microbial metabolites UroA/UAS03 and polysaccharides can promote IL-10 production and mediate anti-inflammatory activities in colon macrophages [68,69]. Moreover, Ifrim et al. also reported that high doses of pattern recognition receptors (PRR) ligands such as flagellin, lipopolysaccharide, and poly I:C, can led to a tolerance of macrophages [70].
2.1.3. Effects of Gut Microbiota on the Other Resident Macrophages
Recent studies have also found the effects of the gut microbiota on perivascular macrophages and muscularis macrophages. Honda et al. [21] demonstrated that a tight anatomical barrier between microbiome and LP perivascular macrophages can prevent bacterial translocation. Gut microbiota alternation with age can change the phenotype of muscularis macrophages (MMs) and affect gastrointestinal motility [71]. Recent data have shown that MMs upregulate neuroprotective factors via β2-adrenergic receptor (β2-AR) signaling [72]. The roles of the gut microbiota in the association between gastrointestinal motility and 5-hydroxytryptophan (HT) expression have also been found in resident macrophages of the gastrointestinal tract [73]. However, long-living tissue-resident macrophages, which express both CD4 and TIM4 molecules, are not affected by the microbiota in adult mice [13].
2.2. Effects of Gut Resident Macrophages on Microbiota
Macrophages are one of the critical cell populations for maintaining intestinal homeostasis [74]. Macrophage depletion using clodronate liposomes can alter the composition of the gut microbiota in the azoxymethane (AOM)/dextran sulfate sodium (DSS) mouse model of colon cancer [75]. In the macrophage-depleted group, increased Firmicutes has been found [75]. Recently, Earley et al. [76] found that intestinal macrophages are important in the normal colonization of gut microbes in adult zebrafish via macrophage-deficient interferon regulatory factor 8 (IRF8).
3. Gut Epithelium and Microbiota
Gut epithelium stands as a single-cell barrier between the intestinal microbiota and the submucosal immune cells, representing the primary shield to prevent microbial translocation. It is formed by various differentiated cell types, including stem cells, enterocytes, goblet cells, Paneth cells, Tuft cells, and M cells [77]. These cells can potentially develop specialized functions such as intestinal enteroendocrine cells that comprise at least eight cellular subsets, including enterochromaffin cells (5-HT/serotonin), D cells (somatostatin), and G cells (gastrin) [78].
3.1. Gut Epithelial Structure and Function Needs Gut Microbiota
3.1.1. Effects of Gut Microbiota on the Gut Epithelial Cells
Gut epithelial structure and function needs in the gut microbiota.
Lactobacillus spp., a kind of generally recognized probiotic, can promote the proliferation of intestinal epithelial cells (IEC) by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-1 dependent reactive oxygen species [79]. Compared with healthy volunteers, F. prausnitzii in patients with inflammatory bowel disease and irritable bowel syndrome is reduced [80,81,82]. Recent studies have shown that F. prausnitzii plays a protective role in the intestinal tissue [83,84,85].
3.1.2. Effects of the Commensal-Derived Metabolites on Gut Epithelial Cells
(1) SCFAs. SCFAs can modulate the proliferation, differentiation, and functions of IEC to impact gut motility and gut barrier functions, as well as host metabolism [86]. Luminal butyrate can serve as a source of energy for gut epithelial cells in metabolic processes, and act to inhibit HDAC activity in intestinal epithelial stem cells [87,88]. SCFAs also affect pro-inflammatory cytokine production through enhancing NF-κB activation in epithelial cells [89]. Goblet cell differentiation can be influenced by acetate, a SCFA produced by Bifidobacterium in a gnotobiotic rodent model [90]. Considerable evidence supports that the concentration of SCFAs is decreased in the colonic lumen of ulcerative colitis (UC) [91]. The dybiosis, which is characterized by decreased butyrate-producing species Eaecalibacterium prausnitizii and Roseburia hominis, is also defined in patients with UC [80]. SCFAs-sensing GPCRs can maintain intestinal epithelial barrier homeostasis via protecting against intestine inflammation. Studies have also found that G protein-coupled receptor (GPR)109a−/− and GPR43−/− mice could suffer from more severe DSS-induced colitis [44]. The administration of SCFAs in UC patients can ameliorate colitis [92]. The SCFA mixture (sodium acetate, sodium propionate, and sodium butyrate) enhances the efficacy of IBD treatments [93].
(2) TRP metabolites. Studies have found that indole derivatives of TRP catabolism can directly stimulate human and mouse Trpa1 and intestinal 5-HT secretion to increase intestinal motility [94]. TRP metabolites indole-3-ethanol, indole-3-pyruvate, and indole-3-aldehyde can impede gut permeability by maintaining the integrity of the apical junctional complex and the associated actin regulatory proteins [95]. TRP also alleviates inflammatory responses to the ameliorate barrier integrity through the CaSR/Rac1/PLC-γ1 signaling pathway to enterotoxigenic Escherichia coli in porcine intestinal epithelial cells [96]. Studies have also found that transepithelial electrical resistance can be increased by indole-3-propionic acid (IPA). IPA also decreases paracellular permeability via the increase in tight junction proteins (claudin-1, occludin, and ZO-1) [97].
(3) Secondary bile acids. The secondary bile acid receptor, TGR5, is expressed in the entire gastrointestinal tract. This receptor regulates a lot of gut homeostatic functions [98,99]. For example, endogenous bile acids in the intestinal lumen can promote intestinal stem cell (ISC) renewal and drive regeneration in response to injury [100]. Notably, bile acid-activated receptors such as FXR, GPBAR1, RORγt, VDR, and PXR have a close relationship with IBD [101]. Studies have found that altered bile acid-activated receptors can be found in patients with IBD. Thus, in these patients with IBD, restoring bile acid signaling might be beneficial [101]. Studies have indicated that the TGR5 ligand can exert profound effects in rodent models of colitis [61].
(4) Other metabolites. Other commensal-derived metabolites are also involved in preserving gut epithelial structure and function, such as polyamines [102]. Epithelial proliferation and macrophage differentiation could be regulated by symbiotic polyamine in the colon [66]. Microbe-derived lactate also is a potent inducer of colonic hyper-proliferation in mice [103]. The microbial metabolites can maintain the homeostasis of gut stem cells via the Wnt/β-catenin pathway [104]. Other signaling pathways, such as the JAK/STAT pathway, are also necessary in the bacteria-modulated epithelium homeostasis [105,106,107]. However, the mechanisms involved in these regulations are too complicated to make a complete interpretation.
3.1.3. Effects of Gut Microbiota on the Gut Organoids
Studies of intestinal epithelial organoids also support that the gut epithelial structure and function needs the gut microbiota. Cryptosporidium parvum infection attenuates ex vivo propagation of murine intestinal enteroids [108]. Lactobacillus accelerates intestinal stem cell regeneration via activation of the signal transducer and activation of the transcription 3 (STAT3) signaling pathway [109]. Akkermansia muciniphila (A. muciniphila), an abundant member of the human gut microbiota can ferment mucus glycoproteins into propionate and acetate in the mucus layer [110]. After exposure to A. muciniphila, mouse gut organoids change the expression of host transcription factors, indicating the effects of A. muciniphila on metabolic activity [111].
3.2. Effects of Gut Epithelial Cells on Gut Microbiota
Gut epithelial cells can produce AMPs, including defensins, C-type lectins such as regenerating islet-derived protein Ⅲγ (Reg IIIγ), and lysozyme [112]. These AMPs target conservative and essential bacterial features and structures. For instance, pore-forming defensins are aimed at the surface membrane, whereas C-type lectins have a strong effect on Gram-positive cell wall peptidoglycans. The antibacterial lectin RegIIIγ promotes the spatial segregation of the microbiota and host in the intestine [113]. Chemokines are shown to possess an antimicrobial activity, including Gram positive and Gram negative bacterial pathogens [114], such as that cys-x-cys ligand 9 (CXCL9) contributes to antimicrobial protection of the gut during Citrobacter rodentium infection, independent of chemokine-receptor signaling [115]. The necleotide-binding oligomerization domian, leucine rich repeat, and pyrin domain conyaining proteins (NLRP9b), which are specifically expressed in intestinal epithelial cells [116], restrict rotavirus infection in intestinal epithelial cells. In addition, inflammasomes can regulate the gut microbiota composition in mice models. The absence of inflammasome components is also related to pathologic alterations in the gut microbiota or dysbiosis [117]. Immunoglobulins A, which is secreted into the lumen, offers immune protection by binding the bacteria or viruses and decreasing their invasion [118]. A recent study identified a highly glycosylated glycosylphosphatidylinositol (GPI)-anchored protein called Ly6/Plaur domain-containing 8 (Lypd8), which contributes to the segregation of intestinal bacteria and intestinal epithelia in the large intestine [119]. Tuft cells, taste-chemosensory epithelial cells, can eliminate parasites by producing IL-25 [120]. These strategies help to keep a balanced microbial quantity and composition [121].
4. Gut Resident Macrophages and Gut Epithelial Cells
Intestinal resident macrophages and IEC not only are geographically close to but also communicate and interact closely with each other. Macrophages act as innate immunological effector cells, and many of their intrinsic responses are bound up with the interpretation of microbiota-derived signals by IEC. A large number of resident macrophages are located close to the epithelial layer and considered to sample and present luminal antigens, phagocytize dead cells and work for a harmonious gut environment [9]. Tissue-resident macrophages can translocate sampled lumenal components to CD103+ tolerogenic dendritic cells, which in turn migrate to the mesenteric lymph nodes to induce Tregs. Recently, CX3CR1+ macrophages have been discovered to directly migrate across the epithelium through paracellular channels [122]. It is also possible that intestinal macrophages uptake IEC to obtain antigens indirectly. This was found in follicle-associated epithelia, which uptake and deliver antigens from the lumen to antigen-presenting cells for the induction of antigen-specific IgA [123]. Notably, gut epithelial cells can also condition macrophages towards the resident phenotype. Furthermore, gut epithelial structure and function also need gut macrophages.
4.1. Gut Epithelial Cells Condition Macrophages towards Resident Phenotype
IEC can condition macrophages towards a tolerogenic phenotype. They control macrophage differentiation by thymic stromal lymphopoietin (TSLP), transforming growth factor-β (TGF-β) and prostaglandinE-2 (PGE-2) [124,125]. Macrophage differentiation can also be regulated by colony-stimulating factor (CSF1). The macrophages are deficient in the mice lacking CAF1 factor (Csf1op/op) or the receptor, CSF1R [126]. The IL-10–IL-10R axis plays a crucial role in conditioning the behavior of macrophages and maintains a non-inflammatory state of macrophages in the mucosa. Gut homeostasis is also supported by the research from humans with function mutations in IL-10R [127]. In the excessive inflammation, semaphoring 7A expressed on the basolateral membrane of IEC is capable to induce IL-10 production by intestinal resident macrophages and ameliorate inflammation in DSS-induced colitis [128]. In the gut lumen, the presence of commensals promotes IEC-derived IL-25 secretion, which reduces IL-23 secretion by macrophage [129]. Converging evidence from human observational studies, population genetics, and studies in mice supports the importance of IL-23 in the mucosal inflammation in the gut in particular [130]. Two intestinal macrophage subsets, CD103+CD11b+ and CD103−CD11b+, are able to sample apoptotic IEC [131]. Although the specific genes and pathways are different among macrophages, a common “suppression of inflammation” signature has been noted [131]. These data address the role of IEC in immunosuppression and homeostatic regulation partially through intestinal macrophages. Studies have also found that local tissue-derived signals can control the functional polarization of resident macrophages [132].
4.2. Gut Epithelial Structure and Function Needs Gut Resident Macrophages
Studies have found that macrophages can worsen tissue injury via producing reactive oxygen species and other toxic mediators. However, they also produce a variety of growth factors to regulate epithelial and endothelial cell proliferation. Macrophages can influence the permeability of the epithelium barrier through IL-6 and NO [133]. Activated macrophages supported tissue repair by up-regulating the expression of IL-3 and IL-4 in a murine model of acute epithelial regeneration in the colon [134]. Macrophages associated with the crypts of Lieberkuehn in the colon can help the proliferation and survival of epithelial progenitor cells [135]. Yeh and colleagues [136] showed that the interaction of bone marrow derived macrophages with intestinal stromal cells is critical for the development of radiation-induced fibrosis in mouse models. After depleting CSF1R-dependent macrophages, Paneth cell differentiation is impaired and the population of Lgr5+ intestinal stem cells is reduced, which further impede goblet cell density and the development of M cells in Peyer’s patches in the murine small intestine [137]. Wnt proteins, which are essential for intestinal epithelial proliferation, can be secreted directly by macrophage derived extracellular vesicles [138]. Depletion of extracellular vesicles from the macrophage conditioned medium can rescue ISCs from radiation lethality [138]. Meanwhile, intestinal macrophages may induce the secretion of the Wnt1 inducible signaling protein 1 in mice [139]. IL-10 produced by macrophages also plays a critical role in recovery from intestinal epithelial injury in animal models [140]. Macrophage-derived IL-10 can result in epithelial cAMP response element-binding protein (CREB) activation and in the production of pro-repair WNT1-inducible signaling protein 1 (WISP-1), which induces epithelial cell proliferation and wound closure [140]. In addition, macrophages are able to induce IL-22 production by innate lymphoid cells (ILC)3, which in turn promotes pSTAT3 signaling in IEC and protects them from intestinal injury in the murine colon [141,142]. IFN also increases the proportion of CCR2-dependent macrophages and interleukin (IL)-22-producing innate lymphoid cells via acting on the intestinal epithelial cells. During infection with the helminth Heligmosomoides polygyrus bakeri, antibodies and helminth induce macrophages to produce Cxcl2 for myofibroblasts to carry out normal tissue repair in the small intestine [143]. In addition, IL-36α, which is mainly expressed by CD14+CD64+ inflammatory macrophages, also contributes to colonic epithelial cell proliferation [144].
5. Conclusions
As discussed above, gut macrophages, epithelium, and microbiota cooperate for inflammation and tolerance, forming a special mucosal network for gut homeostasis. Nevertheless, many aspects of cooperation among the gut macrophages, epithelium, and microbiota remain poorly understood. It will be necessary to further determine how changeable microenvironments, including abundant commensals, different niches, and immune status, give rise to diverse differentiation and functions of the gut macrophages. With advanced technology appearing in recent years, such as single-cell RNA sequencing, we will be able to reveal the macrophage landscape more precisely and comprehensively. IEC can segregate gut microbes and gut immune cells via mucosal barriers, sense signals from both populations, and secrete humoral factors, which maintain gut homeostasis. However, how these gut epithelial cells perform their tasks in different pathogen invasions, susceptible and pathological states, and over-reactive or suppressive immune responses needs to be further understood. The probiotic microbes and immune cells like macrophages support typical epithelial structure and functions, where the exact mechanisms are not completely understood. Given the situation that many gut diseases are associated with aberrant microbiota, impaired epithelium, and inappropriate immune response, more detailed knowledge is required to facilitate new diagnoses and therapies for inflammatory bowel diseases and other relative diseases.
Author Contributions
W.C. designed and wrote the original manuscript. D.L. wrote the manuscript and made figure. C.R. wrote and edited the manuscript. R.Y., C.-K.W. and X.S. wrote, supervised, and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by NSFC grants 91842302, 81970488, 91029736, 9162910, 91442111, and 31570114.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.O’Hara A.M., Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688–693. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tap J., Mondot S., Levenez F., Pelletier E., Caron C., Furet J.P., Ugarte E., Munoz-Tamayo R., Paslier D.L., Nalin R., et al. Towards the human intestinal microbiota phylogenetic core. Environ. Microbiol. 2009;11:2574–2584. doi: 10.1111/j.1462-2920.2009.01982.x. [DOI] [PubMed] [Google Scholar]
- 3.Mazmanian S.K., Liu C.H., Tzianabos A.O., Kasper D.L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Kaiser P., Diard M., Stecher B., Hardt W.D. The streptomycin mouse model for Salmonella diarrhea: Functional analysis of the microbiota, the pathogen’s virulence factors, and the host’s mucosal immune response. Immunol. Rev. 2012;245:56–83. doi: 10.1111/j.1600-065X.2011.01070.x. [DOI] [PubMed] [Google Scholar]
- 5.Lynch S.V., Phimister E.G., Pedersen O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016;375:2369–2379. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- 6.Pickard J.M., Zeng M.Y., Caruso R., Nunez G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017;279:70–89. doi: 10.1111/imr.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashimoto D., Miller J., Merad M. Dendritic cell and macrophage heterogeneity in vivo. Immunity. 2011;35:323–335. doi: 10.1016/j.immuni.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gautier E.L., Shay T., Miller J., Greter M., Jakubzick C., Ivanov S., Helft J., Chow A., Elpek K.G., Gordonov S., et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat. Immunol. 2012;13:1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller P.A., Matheis F., Mucida D. Gut macrophages: Key players in intestinal immunity and tissue physiology. Curr. Opin. Immunol. 2020;62:54–61. doi: 10.1016/j.coi.2019.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aychek T., Jung S. Immunology. The axis of tolerance. Science. 2014;343:1439–1440. doi: 10.1126/science.1252785. [DOI] [PubMed] [Google Scholar]
- 11.Zigmond E., Bernshtein B., Friedlander G., Walker C.R., Yona S., Kim K.W., Brenner O., Krauthgamer R., Varol C., Müller W., et al. Macrophage-restricted interleukin-10 receptor deficiency, but not IL-10 deficiency, causes severe spontaneous colitis. Immunity. 2014;40:720–733. doi: 10.1016/j.immuni.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Bain C.C., Bravo-Blas A., Scott C.L., Perdiguero E.G., Geissmann F., Henri S., Malissen B., Osborne L.C., Artis D., Mowat A.M. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat. Immunol. 2014;15:929–937. doi: 10.1038/ni.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaw T.N., Houston S.A., Wemyss K., Bridgeman H.M., Barbera T.A., Zangerle-Murray T., Strangward P., Ridley A.J.L., Wang P., Tamoutounour S., et al. Tissue-resident macrophages in the intestine are long lived and defined by Tim-4 and CD4 expression. J. Exp. Med. 2018;215:1507–1518. doi: 10.1084/jem.20180019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154:274–284. doi: 10.1016/j.cell.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Peterson L.W., Artis D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014;14:141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 16.Rescigno M. The intestinal epithelial barrier in the control of homeostasis and immunity. Trends Immunol. 2011;32:256–264. doi: 10.1016/j.it.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Kurashima Y., Kiyono H. Mucosal Ecological Network of Epithelium and Immune Cells for Gut Homeostasis and Tissue Healing. Annu. Rev. Immunol. 2017;35:119–147. doi: 10.1146/annurev-immunol-051116-052424. [DOI] [PubMed] [Google Scholar]
- 18.Chtanova T., Han S.J., Schaeffer M., van Dooren G.G., Herzmark P., Striepen B., Robey E.A. Dynamics of T cell, antigen-presenting cell, and pathogen interactions during recall responses in the lymph node. Immunity. 2009;31:342–355. doi: 10.1016/j.immuni.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Schepper S., Verheijden S., Aguilera-Lizarraga J., Viola M.F., Boesmans W., Stakenborg N., Voytyuk I., Schmidt I., Boeckx B., Dierckx de Casterle I., et al. Self-Maintaining Gut Macrophages Are Essential for Intestinal Homeostasis. Cell. 2018;175:400–415.e13. doi: 10.1016/j.cell.2018.07.048. [DOI] [PubMed] [Google Scholar]
- 20.Niess J.H., Brand S., Gu X., Landsman L., Jung S., McCormick B.A., Vyas J.M., Boes M., Ploegh H.L., Fox J.G., et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 21.Honda M., Surewaard B.G.J., Watanabe M., Hedrick C.C., Lee W.Y., Brown K., McCoy K.D., Kubes P. Perivascular localization of macrophages in the intestinal mucosa is regulated by Nr4a1 and the microbiome. Nat. Commun. 2020;11:1329. doi: 10.1038/s41467-020-15068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hadis U., Wahl B., Schulz O., Hardtke-Wolenski M., Schippers A., Wagner N., Muller W., Sparwasser T., Forster R., Pabst O. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011;34:237–246. doi: 10.1016/j.immuni.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 23.De Schepper S., Stakenborg N., Matteoli G., Verheijden S., Boeckxstaens G.E. Muscularis macrophages: Key players in intestinal homeostasis and disease. Cell. Immunol. 2018;330:142–150. doi: 10.1016/j.cellimm.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L., Li Z., Skrzypczynska K.M., Fang Q., Zhang W., O’Brien S.A., He Y., Wang L., Zhang Q., Kim A., et al. Single-Cell Analyses Inform Mechanisms of Myeloid-Targeted Therapies in Colon Cancer. Cell. 2020;181:442–459.e29. doi: 10.1016/j.cell.2020.03.048. [DOI] [PubMed] [Google Scholar]
- 25.Kang B., Alvarado L.J., Kim T., Lehmann M.L., Cho H., He J., Li P., Kim B.H., Larochelle A., Kelsall B.L. Commensal microbiota drive the functional diversification of colon macrophages. Mucosal Immunol. 2020;13:216–229. doi: 10.1038/s41385-019-0228-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chapuy L., Bsat M., Sarkizova S., Rubio M., Therrien A., Wassef E., Bouin M., Orlicka K., Weber A., Hacohen N., et al. Two distinct colonic CD14(+) subsets characterized by single-cell RNA profiling in Crohn’s disease. Mucosal Immunol. 2019;12:703–719. doi: 10.1038/s41385-018-0126-0. [DOI] [PubMed] [Google Scholar]
- 27.Summers K.M., Bush S.J., Hume D.A. Network analysis of transcriptomic diversity amongst resident tissue macrophages and dendritic cells in the mouse mononuclear phagocyte system. PLoS Biol. 2020;18:e3000859. doi: 10.1371/journal.pbio.3000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt F., Dahlke K., Batra A., Keye J., Wu H., Friedrich M., Glauben R., Ring C., Loh G., Schaubeck M., et al. Microbial Colonization in Adulthood Shapes the Intestinal Macrophage Compartment. J. Crohn’s Colitis. 2019;13:1173–1185. doi: 10.1093/ecco-jcc/jjz036. [DOI] [PubMed] [Google Scholar]
- 29.Zhou L., Chu C., Teng F., Bessman N.J., Goc J., Santosa E.K., Putzel G.G., Kabata H., Kelsen J.R., Baldassano R.N., et al. Innate lymphoid cells support regulatory T cells in the intestine through interleukin-2. Nature. 2019;568:405–409. doi: 10.1038/s41586-019-1082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koyama M., Mukhopadhyay P., Schuster I.S., Henden A.S., Hülsdünker J., Varelias A., Vetizou M., Kuns R.D., Robb R.J., Zhang P., et al. MHC Class II Antigen Presentation by the Intestinal Epithelium Initiates Graft-versus-Host Disease and Is Influenced by the Microbiota. Immunity. 2019;51:885–898.e7. doi: 10.1016/j.immuni.2019.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim M., Galan C., Hill A.A., Wu W.J., Fehlner-Peach H., Song H.W., Schady D., Bettini M.L., Simpson K.W., Longman R.S., et al. Critical Role for the Microbiota in CX(3)CR1(+) Intestinal Mononuclear Phagocyte Regulation of Intestinal T Cell Responses. Immunity. 2018;49:151–163.e155. doi: 10.1016/j.immuni.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kikuchi T., Mimura K., Ashizawa M., Okayama H., Endo E., Saito K., Sakamoto W., Fujita S., Endo H., Saito M., et al. Characterization of tumor-infiltrating immune cells in relation to microbiota in colorectal cancers. Cancer Immunol. Immunother. 2020;69:23–32. doi: 10.1007/s00262-019-02433-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li R., Zhou R., Wang H., Li W., Pan M., Yao X., Zhan W., Yang S., Xu L., Ding Y., et al. Gut microbiota-stimulated cathepsin K secretion mediates TLR4-dependent M2 macrophage polarization and promotes tumor metastasis in colorectal cancer. Cell Death Differ. 2019;26:2447–2463. doi: 10.1038/s41418-019-0312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu T., Guo Z., Song X., Liu L., Dong W., Wang S., Xu M., Yang C., Wang B., Cao H. High-fat diet-induced dysbiosis mediates MCP-1/CCR2 axis-dependent M2 macrophage polarization and promotes intestinal adenoma-adenocarcinoma sequence. J. Cell. Mol. Med. 2020;24:2648–2662. doi: 10.1111/jcmm.14984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cuevas-Ramos G., Petit C.R., Marcq I., Boury M., Oswald E., Nougayrede J.P. Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proc. Natl. Acad. Sci. USA. 2010;107:11537–11542. doi: 10.1073/pnas.1001261107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raisch J., Rolhion N., Dubois A., Darfeuille-Michaud A., Bringer M.A. Intracellular colon cancer-associated Escherichia coli promote protumoral activities of human macrophages by inducing sustained COX-2 expression. Lab. Investig. A J. Tech. Methods Pathol. 2015;95:296–307. doi: 10.1038/labinvest.2014.161. [DOI] [PubMed] [Google Scholar]
- 37.Chen T., Li Q., Wu J., Wu Y., Peng W., Li H., Wang J., Tang X., Peng Y., Fu X. Fusobacterium nucleatum promotes M2 polarization of macrophages in the microenvironment of colorectal tumours via a TLR4-dependent mechanism. Cancer Immunol. Immunother. CII. 2018;67:1635–1646. doi: 10.1007/s00262-018-2233-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kles K.A., Chang E.B. Short-chain fatty acids impact on intestinal adaptation, inflammation, carcinoma, and failure. Gastroenterology. 2006;130:S100–S105. doi: 10.1053/j.gastro.2005.11.048. [DOI] [PubMed] [Google Scholar]
- 39.Louis P., Hold G.L., Flint H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014;12:661–672. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- 40.Sun M., Wu W., Liu Z., Cong Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 2017;52:1–8. doi: 10.1007/s00535-016-1242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maldonado Galdeano C., Cazorla S.I., Lemme Dumit J.M., Vélez E., Perdigón G. Beneficial Effects of Probiotic Consumption on the Immune System. Ann. Nutr. Metab. 2019;74:115–124. doi: 10.1159/000496426. [DOI] [PubMed] [Google Scholar]
- 42.Scott N.A., Andrusaite A., Andersen P., Lawson M., Alcon-Giner C., Leclaire C., Caim S., Le Gall G., Shaw T., Connolly J.P.R., et al. Antibiotics induce sustained dysregulation of intestinal T cell immunity by perturbing macrophage homeostasis. Sci. Transl. Med. 2018;10:eaao4755. doi: 10.1126/scitranslmed.aao4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le Poul E., Loison C., Struyf S., Springael J.Y., Lannoy V., Decobecq M.E., Brezillon S., Dupriez V., Vassart G., Van Damme J., et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 2003;278:25481–25489. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 44.Singh N., Gurav A., Sivaprakasam S., Brady E., Padia R., Shi H., Thangaraju M., Prasad P.D., Manicassamy S., Munn D.H., et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–139. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang P.V., Hao L., Offermanns S., Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA. 2014;111:2247–2252. doi: 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luhrs H., Gerke T., Muller J.G., Melcher R., Schauber J., Boxberge F., Scheppach W., Menzel T. Butyrate inhibits NF-kappaB activation in lamina propria macrophages of patients with ulcerative colitis. Scand. J. Gastroenterol. 2002;37:458–466. doi: 10.1080/003655202317316105. [DOI] [PubMed] [Google Scholar]
- 47.Schulthess J., Pandey S., Capitani M., Rue-Albrecht K.C., Arnold I., Franchini F., Chomka A., Ilott N.E., Johnston D.G.W., Pires E., et al. The Short Chain Fatty Acid Butyrate Imprints an Antimicrobial Program in Macrophages. Immunity. 2019;50:432–445.e7. doi: 10.1016/j.immuni.2018.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agus A., Planchais J., Sokol H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe. 2018;23:716–724. doi: 10.1016/j.chom.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 49.Roager H.M., Licht T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018;9:3294. doi: 10.1038/s41467-018-05470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hickman F.W., Steigerwalt A.G., Farmer J.J., 3rd, Brenner D.J. Identification of Proteus penneri sp. nov., formerly known as Proteus vulgaris indole negative or as Proteus vulgaris biogroup 1. J. Clin. Microbiol. 1982;15:1097–1102. doi: 10.1128/jcm.15.6.1097-1102.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dodd D., Spitzer M.H., Van Treuren W., Merrill B.D., Hryckowian A.J., Higginbottom S.K., Le A., Cowan T.M., Nolan G.P., Fischbach M.A., et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature. 2017;551:648–652. doi: 10.1038/nature24661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wlodarska M., Luo C., Kolde R., d’Hennezel E., Annand J.W., Heim C.E., Krastel P., Schmitt E.K., Omar A.S., Creasey E.A., et al. Indoleacrylic Acid Produced by Commensal Peptostreptococcus Species Suppresses Inflammation. Cell Host Microbe. 2017;22:25–37.e6. doi: 10.1016/j.chom.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams B.B., Van Benschoten A.H., Cimermancic P., Donia M.S., Zimmermann M., Taketani M., Ishihara A., Kashyap P.C., Fraser J.S., Fischbach M.A. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe. 2014;16:495–503. doi: 10.1016/j.chom.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cussotto S., Delgado I., Anesi A., Dexpert S., Aubert A., Beau C., Forestier D., Ledaguenel P., Magne E., Mattivi F., et al. Tryptophan Metabolic Pathways Are Altered in Obesity and Are Associated With Systemic Inflammation. Front. Immunol. 2020;11:557. doi: 10.3389/fimmu.2020.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krishnan S., Ding Y., Saedi N., Choi M., Sridharan G.V., Sherr D.H., Yarmush M.L., Alaniz R.C., Jayaraman A., Lee K. Gut Microbiota-Derived Tryptophan Metabolites Modulate Inflammatory Response in Hepatocytes and Macrophages. Cell Rep. 2018;23:1099–1111. doi: 10.1016/j.celrep.2018.03.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kitahara M., Takamine F., Imamura T., Benno Y. Clostridium hiranonis sp. nov., a human intestinal bacterium with bile acid 7alpha-dehydroxylating activity. Int. J. Syst. Evol. Microbiol. 2001;51:39–44. doi: 10.1099/00207713-51-1-39. [DOI] [PubMed] [Google Scholar]
- 57.Ridlon J.M., Kang D.J., Hylemon P.B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 58.Fiorucci S., Biagioli M., Zampella A., Distrutti E. Bile Acids Activated Receptors Regulate Innate Immunity. Front. Immunol. 2018;9:1853. doi: 10.3389/fimmu.2018.01853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Levy M., Thaiss C.A., Elinav E. Metabolites: Messengers between the microbiota and the immune system. Genes Dev. 2016;30:1589–1597. doi: 10.1101/gad.284091.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang G., Huang S., Wang Y., Cai S., Yu H., Liu H., Zeng X., Zhang G., Qiao S. Bridging intestinal immunity and gut microbiota by metabolites. Cell. Mol. Life Sci. 2019;76:3917–3937. doi: 10.1007/s00018-019-03190-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Biagioli M., Carino A., Cipriani S., Francisci D., Marchiano S., Scarpelli P., Sorcini D., Zampella A., Fiorucci S. The Bile Acid Receptor GPBAR1 Regulates the M1/M2 Phenotype of Intestinal Macrophages and Activation of GPBAR1 Rescues Mice from Murine Colitis. J. Immunol. 2017;199:718–733. doi: 10.4049/jimmunol.1700183. [DOI] [PubMed] [Google Scholar]
- 62.Haselow K., Bode J.G., Wammers M., Ehlting C., Keitel V., Kleinebrecht L., Schupp A.K., Haussinger D., Graf D. Bile acids PKA-dependently induce a switch of the IL-10/IL-12 ratio and reduce proinflammatory capability of human macrophages. J. Leukoc. Biol. 2013;94:1253–1264. doi: 10.1189/jlb.0812396. [DOI] [PubMed] [Google Scholar]
- 63.Wang Y.D., Chen W.D., Yu D., Forman B.M., Huang W. The G-protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor kappa light-chain enhancer of activated B cells (NF-kappaB) in mice. Hepatology. 2011;54:1421–1432. doi: 10.1002/hep.24525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pols T.W., Nomura M., Harach T., Lo Sasso G., Oosterveer M.H., Thomas C., Rizzo G., Gioiello A., Adorini L., Pellicciari R., et al. TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab. 2011;14:747–757. doi: 10.1016/j.cmet.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee J.H., Park E., Jin H.J., Lee Y., Choi S.J., Lee G.W., Chang P.S., Paik H.D. Anti-inflammatory and anti-genotoxic activity of branched chain amino acids (BCAA) in lipopolysaccharide (LPS) stimulated RAW 264.7 macrophages. Food Sci. Biotechnol. 2017;26:1371–1377. doi: 10.1007/s10068-017-0165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakamura A., Kurihara S., Takahashi D., Ohashi W., Nakamura Y., Kimura S., Onuki M., Kume A., Sasazawa Y., Furusawa Y., et al. Symbiotic polyamine metabolism regulates epithelial proliferation and macrophage differentiation in the colon. Nat. Commun. 2021;12:2105. doi: 10.1038/s41467-021-22212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kawai Y., Nishikawa T., Shiba Y., Saito S., Murota K., Shibata N., Kobayashi M., Kanayama M., Uchida K., Terao J. Macrophage as a target of quercetin glucuronides in human atherosclerotic arteries: Implication in the anti-atherosclerotic mechanism of dietary flavonoids. J. Biol. Chem. 2008;283:9424–9434. doi: 10.1074/jbc.M706571200. [DOI] [PubMed] [Google Scholar]
- 68.Singh R., Chandrashekharappa S., Bodduluri S.R., Baby B.V., Hegde B., Kotla N.G., Hiwale A.A., Saiyed T., Patel P., Vijay-Kumar M., et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat. Commun. 2019;10:89. doi: 10.1038/s41467-018-07859-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Danne C., Ryzhakov G., Martínez-López M., Ilott N.E., Franchini F., Cuskin F., Lowe E.C., Bullers S.J., Arthur J.S.C., Powrie F. A Large Polysaccharide Produced by Helicobacter hepaticus Induces an Anti-inflammatory Gene Signature in Macrophages. Cell Host Microbe. 2017;22:733–745.e5. doi: 10.1016/j.chom.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ifrim D.C., Quintin J., Joosten L.A., Jacobs C., Jansen T., Jacobs L., Gow N.A., Williams D.L., van der Meer J.W., Netea M.G. Trained immunity or tolerance: Opposing functional programs induced in human monocytes after engagement of various pattern recognition receptors. Clin. Vaccine Immunol. 2014;21:534–545. doi: 10.1128/CVI.00688-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Becker L., Spear E.T., Sinha S.R., Haileselassie Y., Habtezion A. Age-Related Changes in Gut Microbiota Alter Phenotype of Muscularis Macrophages and Disrupt Gastrointestinal Motility. Cell. Mol. Gastroenterol. Hepatol. 2019;7:243–245.e2. doi: 10.1016/j.jcmgh.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matheis F., Muller P.A., Graves C.L., Gabanyi I., Kerner Z.J., Costa-Borges D., Ahrends T., Rosenstiel P., Mucida D. Adrenergic Signaling in Muscularis Macrophages Limits Infection-Induced Neuronal Loss. Cell. 2020;180:64–78.e16. doi: 10.1016/j.cell.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang M., Fukui H., Eda H., Kitayama Y., Hara K., Kodani M., Tomita T., Oshima T., Watari J., Miwa H. Involvement of gut microbiota in the association between gastrointestinal motility and 5HT expression/M2 macrophage abundance in the gastrointestinal tract. Mol. Med. Rep. 2017;16:3482–3488. doi: 10.3892/mmr.2017.6955. [DOI] [PubMed] [Google Scholar]
- 74.Bain C.C., Mowat A.M. Macrophages in intestinal homeostasis and inflammation. Immunol. Rev. 2014;260:102–117. doi: 10.1111/imr.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bader J.E., Enos R.T., Velázquez K.T., Carson M.S., Nagarkatti M., Nagarkatti P.S., Chatzistamou I., Davis J.M., Carson J.A., Robinson C.M., et al. Macrophage depletion using clodronate liposomes decreases tumorigenesis and alters gut microbiota in the AOM/DSS mouse model of colon cancer. Am. J. Physiol. Gastrointest. Liver Physiol. 2018;314:G22–G31. doi: 10.1152/ajpgi.00229.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Earley A.M., Graves C.L., Shiau C.E. Critical Role for a Subset of Intestinal Macrophages in Shaping Gut Microbiota in Adult Zebrafish. Cell Rep. 2018;25:424–436. doi: 10.1016/j.celrep.2018.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barker N. Adult intestinal stem cells: Critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol. 2014;15:19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- 78.Gribble F.M., Reimann F. Enteroendocrine Cells: Chemosensors in the Intestinal Epithelium. Annu. Rev. Physiol. 2016;78:277–299. doi: 10.1146/annurev-physiol-021115-105439. [DOI] [PubMed] [Google Scholar]
- 79.Jones R.M., Luo L., Ardita C.S., Richardson A.N., Kwon Y.M., Mercante J.W., Alam A., Gates C.L., Wu H., Swanson P.A., et al. Symbiotic lactobacilli stimulate gut epithelial proliferation via Nox-mediated generation of reactive oxygen species. EMBO J. 2013;32:3017–3028. doi: 10.1038/emboj.2013.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Machiels K., Joossens M., Sabino J., De Preter V., Arijs I., Eeckhaut V., Ballet V., Claes K., Van Immerseel F., Verbeke K., et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014;63:1275–1283. doi: 10.1136/gutjnl-2013-304833. [DOI] [PubMed] [Google Scholar]
- 81.Pittayanon R., Lau J.T., Leontiadis G.I., Tse F., Yuan Y., Surette M., Moayyedi P. Differences in Gut Microbiota in Patients With vs Without Inflammatory Bowel Diseases: A Systematic Review. Gastroenterology. 2020;158:930–946.e1. doi: 10.1053/j.gastro.2019.11.294. [DOI] [PubMed] [Google Scholar]
- 82.Pittayanon R., Lau J.T., Yuan Y., Leontiadis G.I., Tse F., Surette M., Moayyedi P. Gut Microbiota in Patients With Irritable Bowel Syndrome—A Systematic Review. Gastroenterology. 2019;157:97–108. doi: 10.1053/j.gastro.2019.03.049. [DOI] [PubMed] [Google Scholar]
- 83.Zhou L., Zhang M., Wang Y., Dorfman R.G., Liu H., Yu T., Chen X., Tang D., Xu L., Yin Y., et al. Faecalibacterium prausnitzii Produces Butyrate to Maintain Th17/Treg Balance and to Ameliorate Colorectal Colitis by Inhibiting Histone Deacetylase 1. Inflamm. Bowel Dis. 2018;24:1926–1940. doi: 10.1093/ibd/izy182. [DOI] [PubMed] [Google Scholar]
- 84.Quévrain E., Maubert M.A., Michon C., Chain F., Marquant R., Tailhades J., Miquel S., Carlier L., Bermúdez-Humarán L.G., Pigneur B., et al. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn’s disease. Gut. 2016;65:415–425. doi: 10.1136/gutjnl-2014-307649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hornef M.W., Pabst O. Real friends: Faecalibacterium prausnitzii supports mucosal immune homeostasis. Gut. 2016;65:365–367. doi: 10.1136/gutjnl-2015-310027. [DOI] [PubMed] [Google Scholar]
- 86.Martin-Gallausiaux C., Marinelli L., Blottiere H.M., Larraufie P., Lapaque N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021;80:37–49. doi: 10.1017/S0029665120006916. [DOI] [PubMed] [Google Scholar]
- 87.Donohoe D.R., Garge N., Zhang X., Sun W., O’Connell T.M., Bunger M.K., Bultman S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13:517–526. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kaiko G.E., Ryu S.H., Koues O.I., Collins P.L., Solnica-Krezel L., Pearce E.J., Pearce E.L., Oltz E.M., Stappenbeck T.S. The Colonic Crypt Protects Stem Cells from Microbiota-Derived Metabolites. Cell. 2016;167:1137. doi: 10.1016/j.cell.2016.10.034. [DOI] [PubMed] [Google Scholar]
- 89.Lin M.Y., de Zoete M.R., van Putten J.P., Strijbis K. Redirection of Epithelial Immune Responses by Short-Chain Fatty Acids through Inhibition of Histone Deacetylases. Front. Immunol. 2015;6:554. doi: 10.3389/fimmu.2015.00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wrzosek L., Miquel S., Noordine M.L., Bouet S., Joncquel Chevalier-Curt M., Robert V., Philippe C., Bridonneau C., Cherbuy C., Robbe-Masselot C., et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013;11:61. doi: 10.1186/1741-7007-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vernia P., Gnaedinger A., Hauck W., Breuer R.I. Organic anions and the diarrhea of inflammatory bowel disease. Dig. Dis. Sci. 1988;33:1353–1358. doi: 10.1007/BF01536987. [DOI] [PubMed] [Google Scholar]
- 92.Scheppach W., Sommer H., Kirchner T., Paganelli G.M., Bartram P., Christl S., Richter F., Dusel G., Kasper H. Effect of butyrate enemas on the colonic mucosa in distal ulcerative colitis. Gastroenterology. 1992;103:51–56. doi: 10.1016/0016-5085(92)91094-K. [DOI] [PubMed] [Google Scholar]
- 93.Vernia P., Marcheggiano A., Caprilli R., Frieri G., Corrao G., Valpiani D., Di Paolo M.C., Paoluzi P., Torsoli A. Short-chain fatty acid topical treatment in distal ulcerative colitis. Aliment. Pharmacol. Ther. 1995;9:309–313. doi: 10.1111/j.1365-2036.1995.tb00386.x. [DOI] [PubMed] [Google Scholar]
- 94.Ye L., Bae M., Cassilly C.D., Jabba S.V., Thorpe D.W., Martin A.M., Lu H.Y., Wang J., Thompson J.D., Lickwar C.R., et al. Enteroendocrine cells sense bacterial tryptophan catabolites to activate enteric and vagal neuronal pathways. Cell Host Microbe. 2021;29:179–196.e9. doi: 10.1016/j.chom.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Scott S.A., Fu J., Chang P.V. Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA. 2020;117:19376–19387. doi: 10.1073/pnas.2000047117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu G., Gu K., Wang F., Jia G., Zhao H., Chen X., Wu C., Zhang R., Tian G., Cai J., et al. Tryptophan Ameliorates Barrier Integrity and Alleviates the Inflammatory Response to Enterotoxigenic Escherichia coli K88 Through the CaSR/Rac1/PLC-gamma1 Signaling Pathway in Porcine Intestinal Epithelial Cells. Front. Immunol. 2021;12:748497. doi: 10.3389/fimmu.2021.748497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li J., Zhang L., Wu T., Li Y., Zhou X., Ruan Z. Indole-3-propionic Acid Improved the Intestinal Barrier by Enhancing Epithelial Barrier and Mucus Barrier. J. Agric. Food Chem. 2021;69:1487–1495. doi: 10.1021/acs.jafc.0c05205. [DOI] [PubMed] [Google Scholar]
- 98.Pols T.W., Noriega L.G., Nomura M., Auwerx J., Schoonjans K. The bile acid membrane receptor TGR5 as an emerging target in metabolism and inflammation. J. Hepatol. 2011;54:1263–1272. doi: 10.1016/j.jhep.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Alemi F., Poole D.P., Chiu J., Schoonjans K., Cattaruzza F., Grider J.R., Bunnett N.W., Corvera C.U. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology. 2013;144:145–154. doi: 10.1053/j.gastro.2012.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sorrentino G., Perino A., Yildiz E., El Alam G., Bou Sleiman M., Gioiello A., Pellicciari R., Schoonjans K. Bile Acids Signal via TGR5 to Activate Intestinal Stem Cells and Epithelial Regeneration. Gastroenterology. 2020;159:956–968.e8. doi: 10.1053/j.gastro.2020.05.067. [DOI] [PubMed] [Google Scholar]
- 101.Fiorucci S., Carino A., Baldoni M., Santucci L., Costanzi E., Graziosi L., Distrutti E., Biagioli M. Bile Acid Signaling in Inflammatory Bowel Diseases. Dig. Dis. Sci. 2021;66:674–693. doi: 10.1007/s10620-020-06715-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rooks M.G., Garrett W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016;16:341–352. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rodriguez-Colman M.J., Schewe M., Meerlo M., Stigter E., Gerrits J., Pras-Raves M., Sacchetti A., Hornsveld M., Oost K.C., Snippert H.J., et al. Interplay between metabolic identities in the intestinal crypt supports stem cell function. Nature. 2017;543:424–427. doi: 10.1038/nature21673. [DOI] [PubMed] [Google Scholar]
- 104.Peck B.C.E., Shanahan M.T., Singh A.P., Sethupathy P. Gut Microbial Influences on the Mammalian Intestinal Stem Cell Niche. Stem Cells Int. 2017;2017:5604727. doi: 10.1155/2017/5604727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jiang H., Patel P.H., Kohlmaier A., Grenley M.O., McEwen D.G., Edgar B.A. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Buchon N., Broderick N.A., Chakrabarti S., Lemaitre B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 2009;23:2333–2344. doi: 10.1101/gad.1827009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cronin S.J., Nehme N.T., Limmer S., Liegeois S., Pospisilik J.A., Schramek D., Leibbrandt A., Simoes Rde M., Gruber S., Puc U., et al. Genome-wide RNAi screen identifies genes involved in intestinal pathogenic bacterial infection. Science. 2009;325:340–343. doi: 10.1126/science.1173164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang X.T., Gong A.Y., Wang Y., Chen X., Lim S.S., Dolata C.E., Chen X.M. Cryptosporidium parvum infection attenuates the ex vivo propagation of murine intestinal enteroids. Physiol. Rep. 2016;4:e13060. doi: 10.14814/phy2.13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hou Q., Ye L., Liu H., Huang L., Yang Q., Turner J.R., Yu Q. Lactobacillus accelerates ISCs regeneration to protect the integrity of intestinal mucosa through activation of STAT3 signaling pathway induced by LPLs secretion of IL-22. Cell Death Differ. 2018;25:1657–1670. doi: 10.1038/s41418-018-0070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Derrien M., Collado M.C., Ben-Amor K., Salminen S., de Vos W.M. The Mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl. Environ. Microbiol. 2008;74:1646–1648. doi: 10.1128/AEM.01226-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lukovac S., Belzer C., Pellis L., Keijser B.J., de Vos W.M., Montijn R.C., Roeselers G. Differential modulation by Akkermansia muciniphila and Faecalibacterium prausnitzii of host peripheral lipid metabolism and histone acetylation in mouse gut organoids. mBio. 2014;5:e01438-14. doi: 10.1128/mBio.01438-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vaishnava S., Behrendt C.L., Ismail A.S., Eckmann L., Hooper L.V. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc. Natl. Acad. Sci. USA. 2008;105:20858–20863. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Khomyakova M., Bukmez O., Thomas L.K., Erb T.J., Berg I.A. A methylaspartate cycle in haloarchaea. Science. 2011;331:334–337. doi: 10.1126/science.1196544. [DOI] [PubMed] [Google Scholar]
- 114.Yang D., Chen Q., Hoover D.M., Staley P., Tucker K.D., Lubkowski J., Oppenheim J.J. Many chemokines including CCL20/MIP-3alpha display antimicrobial activity. J. Leukoc. Biol. 2003;74:448–455. doi: 10.1189/jlb.0103024. [DOI] [PubMed] [Google Scholar]
- 115.Reid-Yu S.A., Tuinema B.R., Small C.N., Xing L., Coombes B.K. CXCL9 contributes to antimicrobial protection of the gut during citrobacter rodentium infection independent of chemokine-receptor signaling. PLoS Pathog. 2015;11:e1004648. doi: 10.1371/journal.ppat.1004648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhu S., Ding S., Wang P., Wei Z., Pan W., Palm N.W., Yang Y., Yu H., Li H.B., Wang G., et al. Nlrp9b inflammasome restricts rotavirus infection in intestinal epithelial cells. Nature. 2017;546:667–670. doi: 10.1038/nature22967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen G.Y. Regulation of the gut microbiome by inflammasomes. Free Radic. Biol. Med. 2017;105:35–40. doi: 10.1016/j.freeradbiomed.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Moor K., Diard M., Sellin M.E., Felmy B., Wotzka S.Y., Toska A., Bakkeren E., Arnoldini M., Bansept F., Co A.D., et al. High-avidity IgA protects the intestine by enchaining growing bacteria. Nature. 2017;544:498–502. doi: 10.1038/nature22058. [DOI] [PubMed] [Google Scholar]
- 119.Okumura R., Kurakawa T., Nakano T., Kayama H., Kinoshita M., Motooka D., Gotoh K., Kimura T., Kamiyama N., Kusu T., et al. Lypd8 promotes the segregation of flagellated microbiota and colonic epithelia. Nature. 2016;532:117–121. doi: 10.1038/nature17406. [DOI] [PubMed] [Google Scholar]
- 120.Gerbe F., Sidot E., Smyth D.J., Ohmoto M., Matsumoto I., Dardalhon V., Cesses P., Garnier L., Pouzolles M., Brulin B., et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature. 2016;529:226–230. doi: 10.1038/nature16527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bevins C.L., Salzman N.H. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Microbiol. 2011;9:356–368. doi: 10.1038/nrmicro2546. [DOI] [PubMed] [Google Scholar]
- 122.Man A.L., Gicheva N., Regoli M., Rowley G., De Cunto G., Wellner N., Bassity E., Gulisano M., Bertelli E., Nicoletti C. CX3CR1+ Cell-Mediated Salmonella Exclusion Protects the Intestinal Mucosa during the Initial Stage of Infection. J. Immunol. 2017;198:335–343. doi: 10.4049/jimmunol.1502559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mabbott N.A., Donaldson D.S., Ohno H., Williams I.R., Mahajan A. Microfold (M) cells: Important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 2013;6:666–677. doi: 10.1038/mi.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat. Rev. Immunol. 2008;8:411–420. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- 125.Gross M., Salame T.M., Jung S. Guardians of the Gut-Murine Intestinal Macrophages and Dendritic Cells. Front. Immunol. 2015;6:254. doi: 10.3389/fimmu.2015.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dai X.M., Ryan G.R., Hapel A.J., Dominguez M.G., Russell R.G., Kapp S., Sylvestre V., Stanley E.R. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002;99:111–120. doi: 10.1182/blood.V99.1.111. [DOI] [PubMed] [Google Scholar]
- 127.Shouval D.S., Biswas A., Goettel J.A., McCann K., Conaway E., Redhu N.S., Mascanfroni I.D., Al Adham Z., Lavoie S., Ibourk M., et al. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity. 2014;40:706–719. doi: 10.1016/j.immuni.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kang S., Okuno T., Takegahara N., Takamatsu H., Nojima S., Kimura T., Yoshida Y., Ito D., Ohmae S., You D.J., et al. Intestinal epithelial cell-derived semaphorin 7A negatively regulates development of colitis via αvβ1 integrin. J. Immunol. 2012;188:1108–1116. doi: 10.4049/jimmunol.1102084. [DOI] [PubMed] [Google Scholar]
- 129.Zaph C., Du Y., Saenz S.A., Nair M.G., Perrigoue J.G., Taylor B.C., Troy A.E., Kobuley D.E., Kastelein R.A., Cua D.J., et al. Commensal-dependent expression of IL-25 regulates the IL-23-IL-17 axis in the intestine. J. Exp. Med. 2008;205:2191–2198. doi: 10.1084/jem.20080720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Moschen A.R., Tilg H., Raine T. IL-12, IL-23 and IL-17 in IBD: Immunobiology and therapeutic targeting. Nat. Rev. Gastroenterol. Hepatol. 2019;16:185–196. doi: 10.1038/s41575-018-0084-8. [DOI] [PubMed] [Google Scholar]
- 131.Cummings R.J., Barbet G., Bongers G., Hartmann B.M., Gettler K., Muniz L., Furtado G.C., Cho J., Lira S.A., Blander J.M. Different tissue phagocytes sample apoptotic cells to direct distinct homeostasis programs. Nature. 2016;539:565–569. doi: 10.1038/nature20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Okabe Y., Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell. 2014;157:832–844. doi: 10.1016/j.cell.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Du Plessis J., Vanheel H., Janssen C.E., Roos L., Slavik T., Stivaktas P.I., Nieuwoudt M., van Wyk S.G., Vieira W., Pretorius E., et al. Activated intestinal macrophages in patients with cirrhosis release NO and IL-6 that may disrupt intestinal barrier function. J. Hepatol. 2013;58:1125–1132. doi: 10.1016/j.jhep.2013.01.038. [DOI] [PubMed] [Google Scholar]
- 134.Seno H., Miyoshi H., Brown S.L., Geske M.J., Colonna M., Stappenbeck T.S. Efficient colonic mucosal wound repair requires Trem2 signaling. Proc. Natl. Acad. Sci. USA. 2009;106:256–261. doi: 10.1073/pnas.0803343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pull S.L., Doherty J.M., Mills J.C., Gordon J.I., Stappenbeck T.S. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc. Natl. Acad. Sci. USA. 2005;102:99–104. doi: 10.1073/pnas.0405979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yeh M.H., Chang Y.H., Tsai Y.C., Chen S.L., Huang T.S., Chiu J.F., Ch’ang H.J. Bone marrow derived macrophages fuse with intestine stromal cells and contribute to chronic fibrosis after radiation. Radiother. Oncol. 2016;119:250–258. doi: 10.1016/j.radonc.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 137.Sehgal A., Donaldson D.S., Pridans C., Sauter K.A., Hume D.A., Mabbott N.A. The role of CSF1R-dependent macrophages in control of the intestinal stem-cell niche. Nat. Commun. 2018;9:1272. doi: 10.1038/s41467-018-03638-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Saha S., Aranda E., Hayakawa Y., Bhanja P., Atay S., Brodin N.P., Li J., Asfaha S., Liu L., Tailor Y., et al. Macrophage-derived extracellular vesicle-packaged WNTs rescue intestinal stem cells and enhance survival after radiation injury. Nat. Commun. 2016;7:13096. doi: 10.1038/ncomms13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Quiros M., Nishio H., Neumann P.A., Siuda D., Brazil J.C., Azcutia V., Hilgarth R., O’Leary M.N., Garcia-Hernandez V., Leoni G., et al. Macrophage-derived IL-10 mediates mucosal repair by epithelial WISP-1 signaling. J. Clin. Investig. 2017;127:3510–3520. doi: 10.1172/JCI90229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Morhardt T.L., Hayashi A., Ochi T., Quiros M., Kitamoto S., Nagao-Kitamoto H., Kuffa P., Atarashi K., Honda K., Kao J.Y., et al. IL-10 produced by macrophages regulates epithelial integrity in the small intestine. Sci. Rep. 2019;9:1223. doi: 10.1038/s41598-018-38125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yang F.C., Chiu P.Y., Chen Y., Mak T.W., Chen N.J. TREM-1-dependent M1 macrophage polarization restores intestinal epithelium damaged by DSS-induced colitis by activating IL-22-producing innate lymphoid cells. J. Biomed. Sci. 2019;26:46. doi: 10.1186/s12929-019-0539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Neil J.A., Matsuzawa-Ishimoto Y., Kernbauer-Hölzl E., Schuster S.L., Sota S., Venzon M., Dallari S., Galvao Neto A., Hine A., Hudesman D., et al. IFN-I and IL-22 mediate protective effects of intestinal viral infection. Nat. Microbiol. 2019;4:1737–1749. doi: 10.1038/s41564-019-0470-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Esser-von Bieren J., Volpe B., Sutherland D.B., Burgi J., Verbeek J.S., Marsland B.J., Urban J.F., Jr., Harris N.L. Immune antibodies and helminth products drive CXCR2-dependent macrophage-myofibroblast crosstalk to promote intestinal repair. PLoS Pathog. 2015;11:e1004778. doi: 10.1371/journal.ppat.1004778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Scheibe K., Backert I., Wirtz S., Hueber A., Schett G., Vieth M., Probst H.C., Bopp T., Neurath M.F., Neufert C. IL-36R signalling activates intestinal epithelial cells and fibroblasts and promotes mucosal healing in vivo. Gut. 2017;66:823–838. doi: 10.1136/gutjnl-2015-310374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.