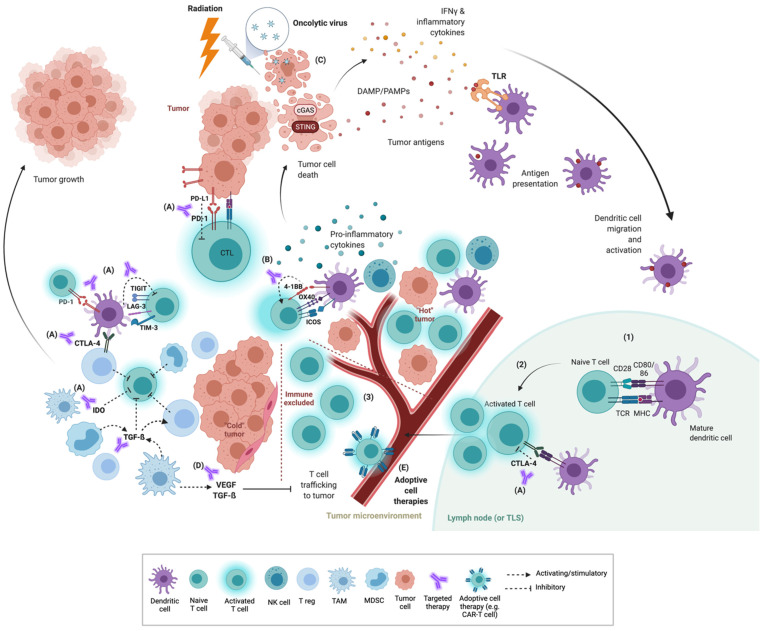
Figure 1.
T-cell tumor response and interactions within the tumor immune microenvironment (TIME). Priming of T cells begins with presentation of tumor antigen to naïve T cells via antigen-presenting cells within draining lymph nodes (and potentially in tumor lymphoid structures, TLS) (1). Activation occurs when T-cell receptors (TCR) recognize their specific peptide antigen presented by MHC and in the presence of co-stimulation signaling between CD28 and CD80/86, with additional co-stimulation from inflammatory cytokines (2). Activated T cells undergo expansion and then traffic to the tumor where they can engage in direct cytotoxicity against tumor cells presenting cognate antigen via MHC. Tumor immune microenvironments can be conceptualised as “hot”, “cold” or “immune excluded” (3). “Hot” or inflamed TIME contain abundant CTLs and other immune cell populations (dendritic cells, NK cells) associated with anti-tumor immunity and expression of inhibitory checkpoints, and thus are more likely to respond to checkpoint inhibition resulting in tumor cell death. “Cold” tumors by contrast contain more immunosuppressive cell populations such as Tregs, TAMs, MDSCs. Immune-excluded tumors contain inflammatory immune infiltrate which is unable to penetrate into the TIME due to stromal populations and secreted factors such as TGF-β and VEGF. These latter two tumor environments are poorly responsive to checkpoint inhibition alone and new combination therapies are being investigated. Immunotherapeutic approaches include (A) blockade of inhibitory checkpoints, (B) stimulation of co-stimulatory checkpoints, (C) priming strategies to increase effective T-cell activation through stimulation of the innate immune system (e.g., radiation, oncolytic viral therapy and STING pathway agonists), (D) targeting immunomodulators (e.g., VEGF, TGF-β) and (E) introduction of primed or engineered T cells via adoptive cell therapy. CTL, cytotoxic T lymphocyte; DAMP, damage-associated molecular patterns; IDO, indole 2,3-dioxygenase; LAG-3, lymphocyte-activation gene 3; MDSC, myeloid-derived suppressor cell; MHC, major histocompatibility complex; PAMP, pathogen-associated molecular patterns; STING, stimulator of interferon genes; TAM, tumor-associated macrophage; TGF-β, transforming growth factor beta; TIGIT, T-cell immunoreceptor with Ig and ITIM domains; TIM-3, T-cell immunoglobulin and mucin-domain-containing molecule 3; TLR, Toll-like receptor; VEGF, vascular endothelial growth factor. Adapted from “Tumor-Specific T cell Induction and Function”, by BioRender.com (2020). Retrieved from https://app.biorender.com/biorender-templates, accessed on 10 September 2021.