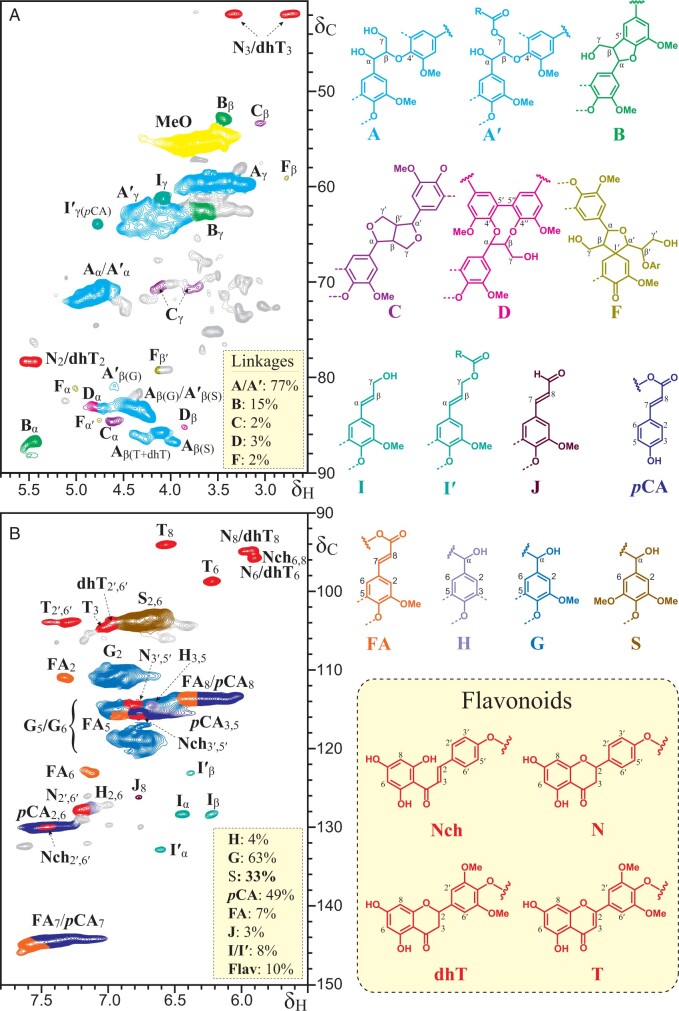
Figure 3.
2D HSQC NMR spectrum (in DMSO-d6) of the MWL preparation isolated from papyrus rind. A, Aliphatic-oxygenated (δC/δH 40 − 90/2.5 − 5.8) and B, aromatic (δC/δH 90 − 150/5.5 − 7.8) regions. The main structures found are as follows: A: β–O–4′ alkyl-aryl ethers; A′: β–O–4′ alkyl-aryl ethers acylated at the γ-OH; B: β–5′ phenylcoumarans; C: β–β′ resinols; D: 5–5′ dibenzodioxocins; F: β–1′ spirodienones; I: p-hydroxycinnamyl alcohol end-groups; I′: p-hydroxycinnamyl alcohol end-groups acylated at the γ-OH; J: p-hydroxycinnamaldehyde end-groups; pCA: p-coumarates; FA: ferulates; H: p-hydroxyphenyl units; G: guaiacyl units; S: syringyl units; Nch: naringenin chalcone; N: naringenin; dhT: dihydritricin; T: tricin. The relative abundances of the different H-, G-, and S-lignin units, inter-unit linkages and p-hydroxycinnamyl end-groups, p-hydroxycinnamates, and flavonoids (Flav = Nch + N + dhT + T), estimated from the integration of the signals in the HSQC spectrum are shown in appropriate boxes. Lignin inter-unit linkages (A/A′, B, C, D, and F) are expressed as fractions of the total linkages types, whereas pCA, FA, J, I/I′ and Flav contents are reported as percentages of the sum of the lignin units (H + G + S = 100).