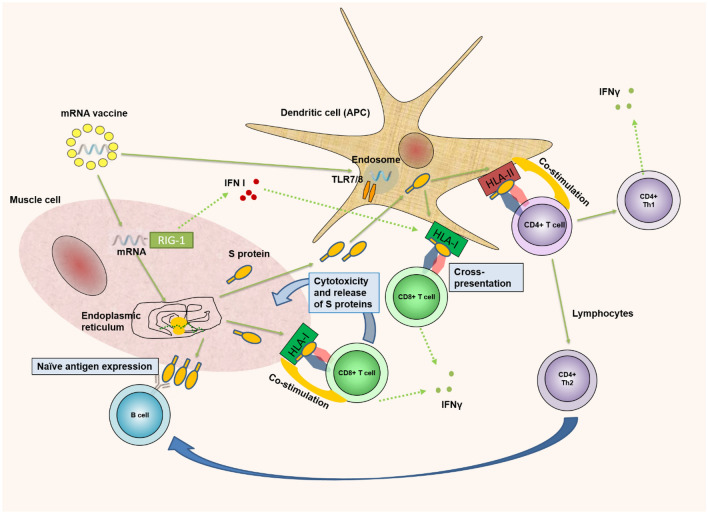
Fig. 1.
Antigen-specific immune response after vaccination to SARS-COV-2. After intramuscular vaccination, mRNA/LNP(lipid nanoparticles) enters myocytes. There, LNPs are degrading to release the mRNA. It enters the endoplasmic reticulum, leading to translation of the mRNA molecule by the ribosomes and generation of the SARS-CoV-2 spike protein. It has the fate of an endogenous antigen that is bound to the HLA-I molecule and presented on the cell surface. Recognition of Tcytotoxic(CD8 + Tc) cells leads to cytotoxic destruction of myocytes. A certain amount of the produced spike protein is secreted outside the myocytes. Spike protein enters the extracellular space. It is absorbed by dendritic cells (DCs) and acts as an exogenous antigen, presented to T-helper 1 and T-helper 2 (CD4 +) cells. Myocyte mRNA activates interferon types I (IFN-I), which enhances the ability of DCs to cross-present exogenous HLA-I epitopes at Tc cells. We have to mention also that methylpseudouridine supports the linear structure of vaccine mRNA and thus, reduces immunogeneicity of vaccine because of TLR7 and RIG-1 [10]. Thick green arrows—proceed; green dot arrow—secretion; blue arrow—influence/help. APC antigen-presenting cell, TLR toll-like receptor, HLA human leukocyte antigen, IFNγ interferon-gamma, Th1 T helper cell 1, Th2 T helper cell 2, RIG 1 retinoic acid-inducible gene I, IFN-I interferon-gamma type one