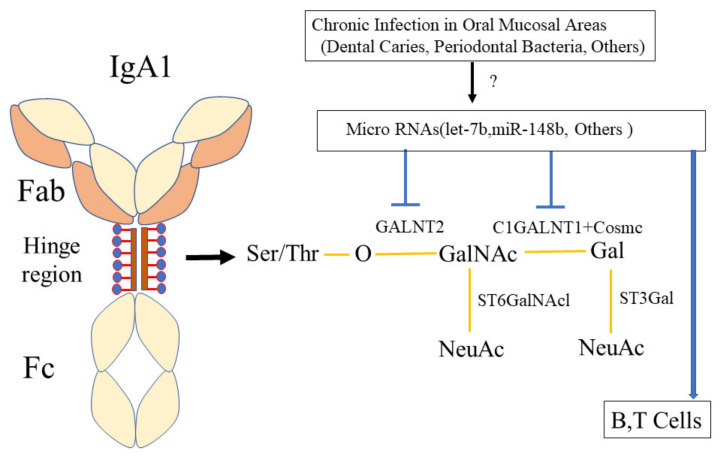
Figure 7.
Hypothesis of the mechanism by which oral bacterial infection causes aberrantly glycosylated IgA1. The hinge region of IgA1 contains serine (Ser) or threonine (Thr) amino acids, which can be glycosylated. This glycosylation contributes to the aggregation of IgA1 because of the reactivity of IgA1 to jacalin, a lectin specific for O-linked glycan side chains containing GalNAc, N-acetyl-galactosamine (GalNAc) and galactose (Gal) linked by beta-1,3-glycosidic bonds. These O-glycan chains in the IgA1 hinge region are formed by sequential reactions that are catalyzed by some glycosyltransferases, such as GALNT2, C1GALT1, and COSMC. The expression levels of these enzymes were altered by microRNAs, such as let-7b and miR-148b, resulting in aberrantly glycosylated IgA1. The expression of these microRNAs might be altered by chronic infections in the oral mucosal areas, such as bacteria related to dental caries and periodontal bacteria. Ser: serine amino acid, Thr: threonine amino acid, GalNAc: N-acetyl-galactosamine, GALNT2: UDP-N-acetyl-alpha-D-galactosamine:polypeptide N-acetyl-galactosaminyl-transferase 2, C1GALT1: core 1 beta-1,3-galactosyltransferase, COSMC: core 1-β3 galactosyl-transferase specific molecular chaperone.