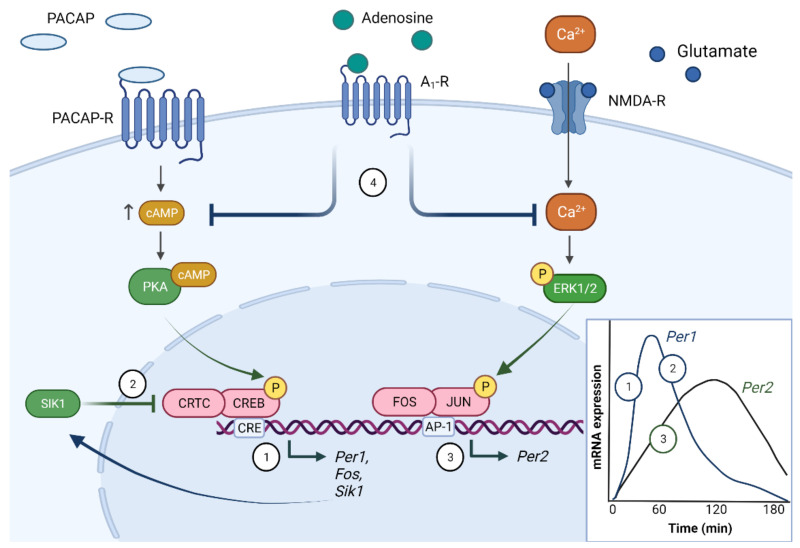
Figure 3.
Molecular photoentrainment of the SCN. The light-induced release of glutamate and pituitary adenylate cyclase-activating polypeptide (PACAP) from the RHT nerve terminals leads to a rise in intracellular Ca2+ and cAMP levels in the SCN. These trigger a cascade of events including activation of protein kinase A (PKA), which activates the transcription factor cAMP response element-binding protein (CREB), together with co-activators such as CREB-regulated transcription coactivator 1 (CRTC1). This leads to the upregulation of CRE-driven genes, including the core clock component, Per1 (1). In addition, Sik1 is upregulated, which feedbacks on the CREB pathway by phosphorylation of CRTC1. This deactivates CRTC1 leading to a decline in CREB-induced gene transcription and therefore a decline in Per1 expression (2). In parallel, the activation of ERK1/2 by Ca2+ influx leads to the upregulation of the immediate-early transcription factors JUN and FOS. These heterodimerise to form AP-1, which drives Per2 transcription leading to an increase in Per2 expression (3). Adenosine, which accumulates in the extracellular space during wakefulness, modulates these light-activated signalling pathways. Adenosine predominantly signals through the Gi (inhibitory) coupled A1 receptor in the SCN, which results in a decrease in intracellular cAMP and Ca2+ levels, and therefore a downregulation of the subsequent signalling events (4). Figure created with BioRender.com.