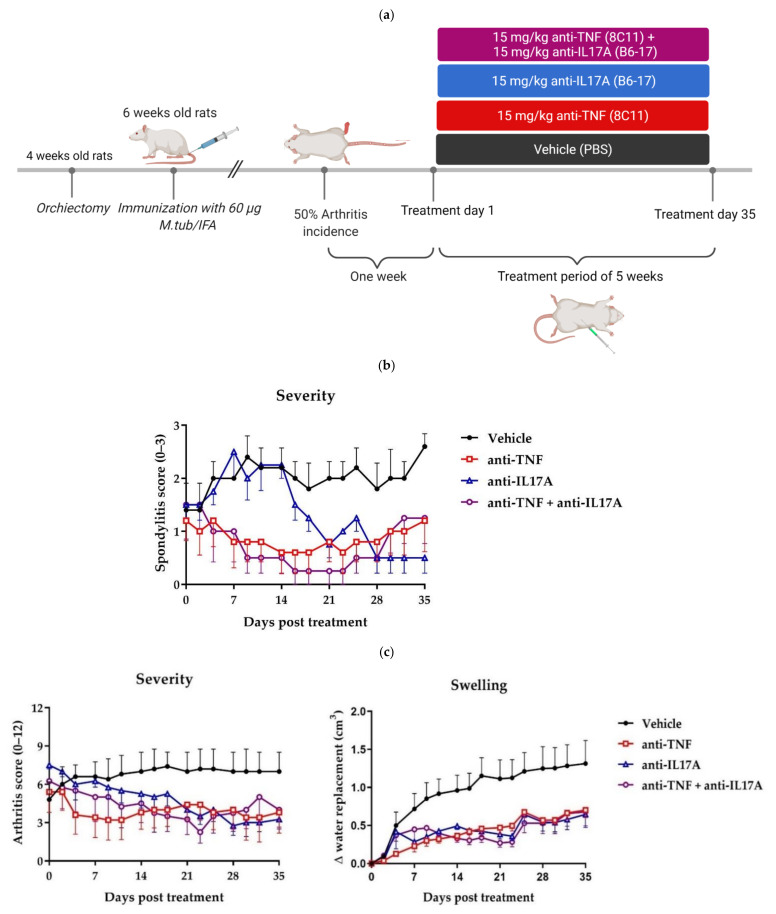
Figure 1.
Tumor necrosis factor (TNF) and interleukin-17A (IL-17A) dual blockade therapy reduces axial and peripheral clinical disease. (a) Study design. Male HLA-B27/huβ2m transgenic rats were orchiectomized at four weeks of age and immunized with 60 µg Mycobacterium tuberculosis (M. tub.) at six weeks of age. One week after arthritis incidence reached 50%, the rats were randomized according to arthritis severity to be treated twice weekly with intraperitoneal injections with either 15 mg/kg anti-TNF and 15 mg/kg anti-IL-17A (8C11 and B6-17, n = 4), 15 mg/kg anti-TNF (8C11, n = 5), 15 mg/kg anti-IL-17A (B6-17, n = 4), or PBS as vehicle control (n = 5) for five weeks. An experimentally blinded observer scored spondylitis severity (0–3 per tail), and peripheral arthritis severity (0–3 per paw) and hind paw swelling (in cm3 water replacement using plethysmography) at multiple time points. (b) Spondylitis severity. A significant decrease was seen for spondylitis score for dual blockade therapy (p = 0.031) and anti-TNF (p = 0.031) versus vehicle; a trend was seen for anti-IL-17A versus vehicle (p = 0.165). (c) Peripheral arthritis severity and hind paw swelling. Numerical, but non-significant, decreases were seen for the combinational and both single therapy groups compared to the vehicle. Hind paw swelling was significantly decreased for combinational (p = 0.008), anti-TNF (p = 0.006) and anti-IL-17A (p = 0.011) treatments compared to vehicle control. Figure (a) was created with BioRender.com.