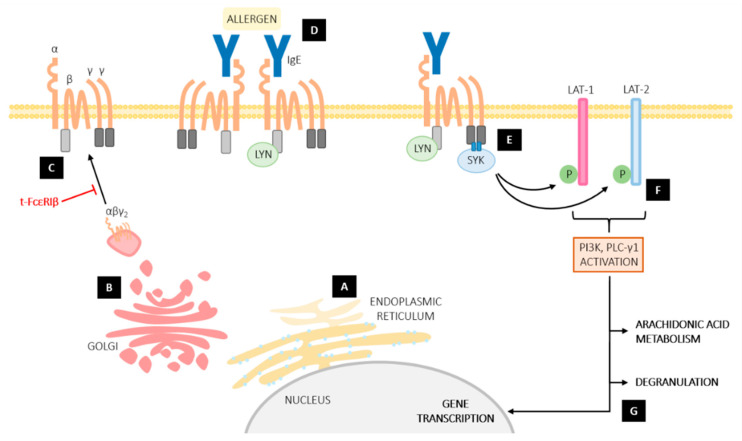
Figure 1.
The role of FcεRIβ in mast cell signaling pathways. (A) Synthesis of FcεRI α, β and γ-subunits takes place within the endoplasmic reticulum. FcεRIβ facilitates appropriate glycosylation and folding of FcεRIα, and the γ-subunits permit export of the αβγ2 tetrameric complex to the Golgi. (B) Full-length FcεRIβ traffics the receptor complex to the cell surface, whereas t-FcεRIβ, which is incapable of trafficking to the surface, prevents surface expression of FcεRI. (C) Once at the surface, full-length FcεRIβ stabilizes the receptor complex. (D) Binding of IgE to the receptor increases receptor half-life at the surface. Allergen binding cross-links multiple FcεRI and induces receptor aggregation, which leads to phosphorylation of FcεRIβ by LYN. (E) By binding LYN, FcεRIβ amplifies phosphorylation of the FcεRIγ ITAMs, which leads to the recruitment and phosphorylation of SYK. (F) Phosphorylated SYK propagates intracellular signals by phosphorylating LAT and LAT2, which subsequently induce PI3K and PLC-γ1 signaling cascades. (G) Ultimately, these signaling pathways culminate in proinflammatory gene expression and the release of cytokines and chemokines, arachidonic acid metabolism and eicosanoid production, and mediator release via degranulation. By preventing trafficking of FcεRI to the surface, t-FcεRIβ inhibits the downstream cellular events of SYK phosphorylation, including mast cell mediator release. LYN, SRC family protein tyrosine kinase; ITAM, immunoreceptor tyrosine-based activation motifs; SYK, spleen tyrosine kinase; LAT-1, linker for activation of T cells; LAT-2, LAT-1 related adaptor; PI3K, phosphatidylinositol 3-kinase; PLC-γ1, phospholipase C-γ1.