Figure 1 -. Overview of LNP library design, formulation, and ex utero screening in amniotic fluids to predict intra-amniotic delivery.
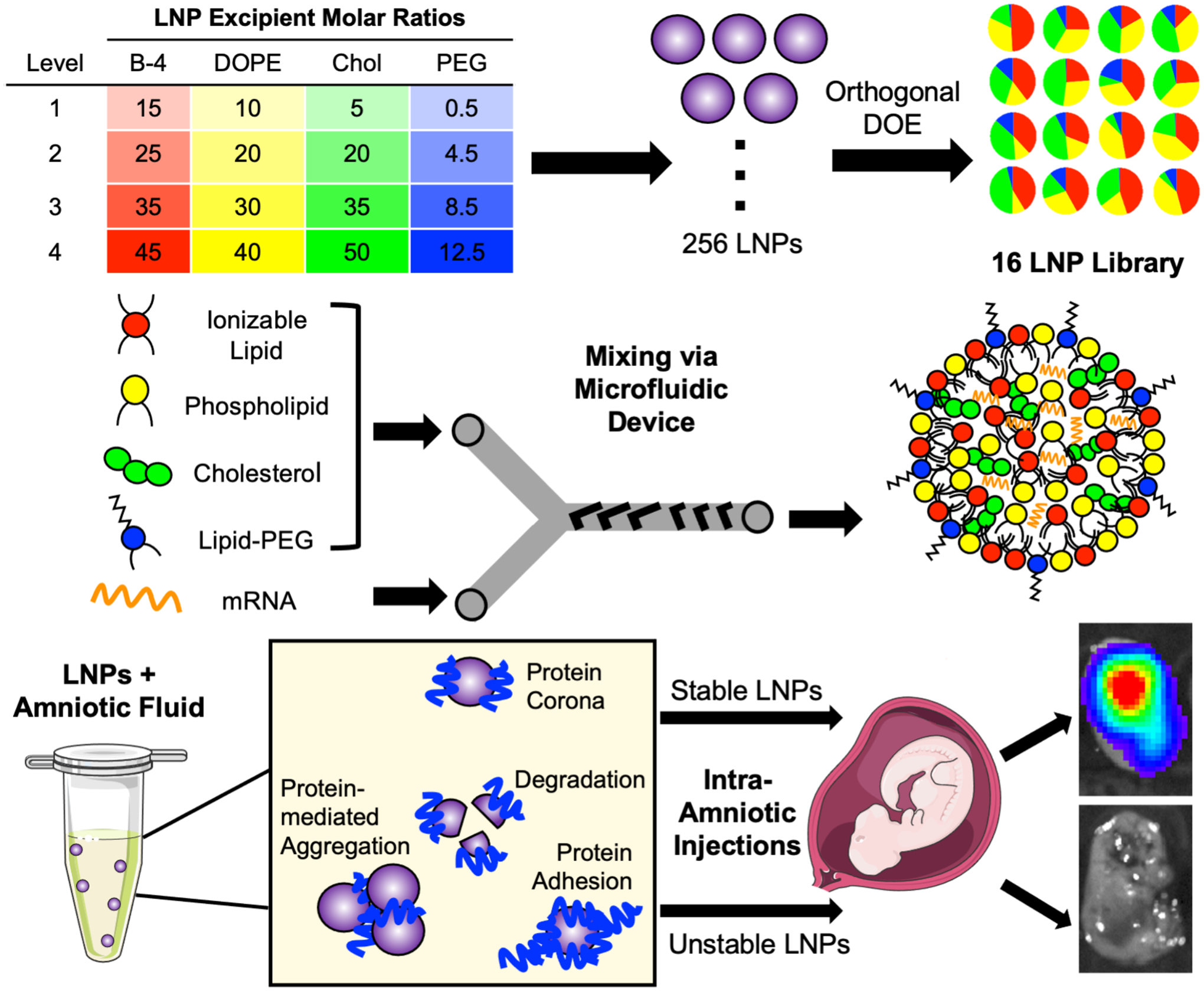
A library of 16 LNP formulations was generated using orthogonal design of experiments (DOE) to explore four molar ratios of each of four excipients. Next, each LNP was synthesized by combining an ethanol phase of lipid excipients – including ionizable lipid, DOPE phospholipid, cholesterol, and lipid-PEG – with an aqueous phase containing luciferase mRNA. The two phases were mixed at controlled flow rates in a microfluidic device to form LNPs. Then, LNPs were screened ex utero in fetal fluids to identify stable and unstable LNPs for intra-amniotic delivery.
