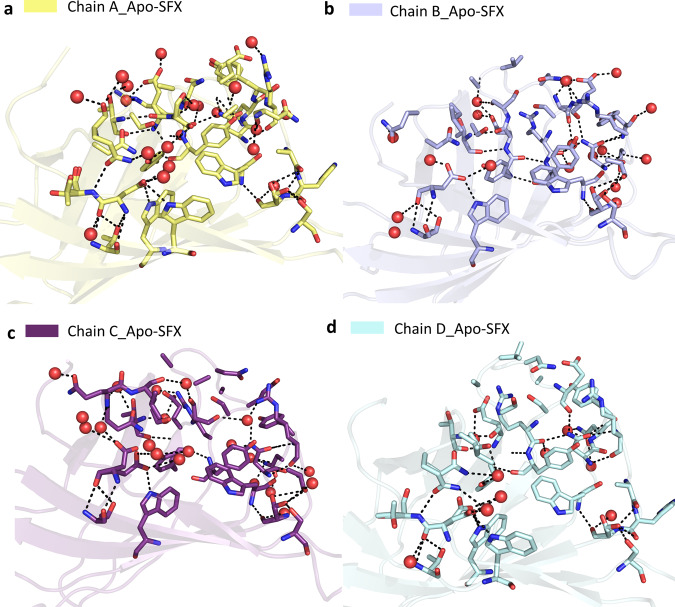
Fig. 5. Representation of coordinated water molecules and polar interactions near the binding sites for each chain of Apo-SFX structure.
Coordinated water molecules within the binding pocket were altered and polar interactions were reduced with loop opening in Apo-SFX structure. All polar interactions were observed within 3.6 Å. a Binding site residues of chain A were observed with 19 water molecules and 51 polar interactions. Those interactions provide the stability of the chain, which was similar to the selenobiotin-bounded structure. b Chain B-binding site residues were determined with 19 water molecules and 46 polar interactions that were involved. c Residues of the binding site of chain C have 40 polar interactions between residues and 20 water molecules. d Chain D-binding site residues involve 9 water molecules and 31 polar interactions. All interactions included H-bonds and electrostatic interactions and are presented with dashed lines. Water molecules are indicated with red-colored spheres.