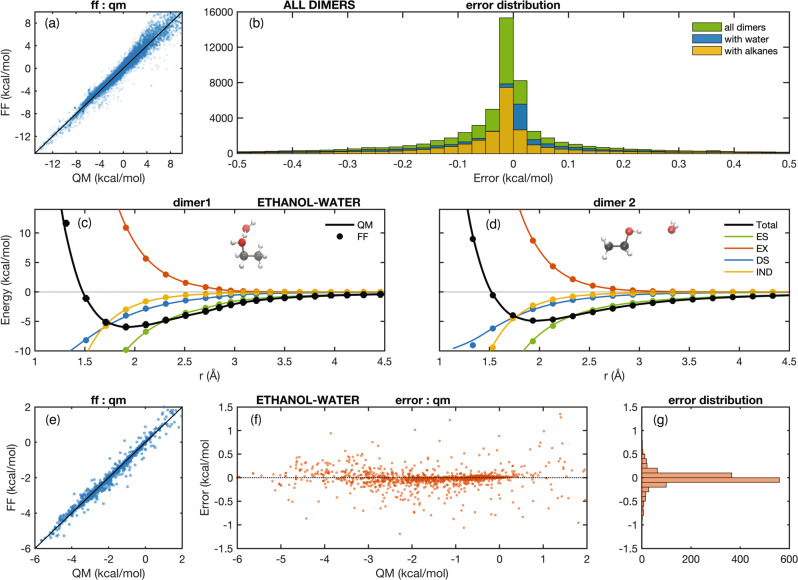
Fig. 1. QM: FF energies’ correspondences and deviations.
a FF vs. QM energies for all the dimers in our training sets. The functional form reproduces the lower energy conformations very well and is designed to permit a larger error in less important high-energy high electron overlap regions. b the distribution of errors for our training dimer sets. The MAE of errors are 0.17 kcal/mol for all, 0.19 kcal/mol for dimers with water (total number of dimers = 36,309), and 0.16 kcal/mol for dimers with alkanes (total number of dimers = 25,986): A specific system (ethanol-water) provides a more detailed illustration of model energies and their correspondence with QM. c, d dissociation curves for primary (c) and secondary (d) minima of the ethanol-water dimer. QM energies are solid lines and FF values are filled circles. The colors designate the energy components: electrostatics (ES), exchange-repulsion (EX), dispersion (DS) and induction (IND). The agreements for the total energy and for each component are excellent. e–g Error distributions for the ethanol-water dimer: e is analogous to a; f is a difference plot offering a more detailed view and is projected onto (g) the error distribution. The MAE for the errors in this system is 0.08 kcal/mol.